当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Ligand-Controlled Enantioselective and Regiodivergent Construction of 1,2- and 1,3-Disubstituted Alicycles
Organic Letters ( IF 4.9 ) Pub Date : 2024-09-10 , DOI: 10.1021/acs.orglett.4c02975 Ke Zeng 1, 2 , Ludi Li 3 , Xiaoyao Luo 1 , Yue Wang 2 , Xiaorong Song 2 , Pengli Zhang 2 , Guoqin Xia 2
Organic Letters ( IF 4.9 ) Pub Date : 2024-09-10 , DOI: 10.1021/acs.orglett.4c02975 Ke Zeng 1, 2 , Ludi Li 3 , Xiaoyao Luo 1 , Yue Wang 2 , Xiaorong Song 2 , Pengli Zhang 2 , Guoqin Xia 2
Affiliation
|
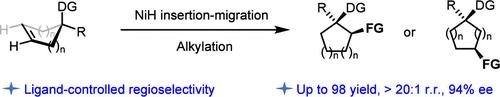
Three-dimensional alicyclic skeletons with multiple stereochemically defined chiral centers are highly valuable in modern drug discovery. Here, we reported a diverse approach to access 1,2- and 1,3-disubstituted chiral cycloalkanes by the strategy of NiH-catalyzed, transannular-directed alkene desymmetrization. The ring strain of the bridged bicyclic organonickel intermediate and the coordination effect of the ligand were identified as crucial factors in determining site selectivity by influencing the NiH migration step. This methodology demonstrates a broad substrate scope and displays good tolerance toward various functional groups, resulting in excellent outcomes in terms of the yield, regioselectivity, and enantioselectivity.
中文翻译:

配体控制的 1,2- 和 1,3-二取代脂环化合物的对映选择性和区域发散构建
具有多个立体化学定义的手性中心的三维脂环骨架在现代药物发现中非常有价值。在这里,我们报道了通过 NiH 催化、跨环定向烯烃去对称化策略获得 1,2- 和 1,3-二取代手性环烷烃的多种方法。桥联双环有机镍中间体的环应变和配体的配位效应被认为是通过影响 NiH 迁移步骤来确定位点选择性的关键因素。该方法展示了广泛的底物范围,并对各种官能团表现出良好的耐受性,在产率、区域选择性和对映选择性方面产生了优异的结果。
更新日期:2024-09-10
中文翻译:

配体控制的 1,2- 和 1,3-二取代脂环化合物的对映选择性和区域发散构建
具有多个立体化学定义的手性中心的三维脂环骨架在现代药物发现中非常有价值。在这里,我们报道了通过 NiH 催化、跨环定向烯烃去对称化策略获得 1,2- 和 1,3-二取代手性环烷烃的多种方法。桥联双环有机镍中间体的环应变和配体的配位效应被认为是通过影响 NiH 迁移步骤来确定位点选择性的关键因素。该方法展示了广泛的底物范围,并对各种官能团表现出良好的耐受性,在产率、区域选择性和对映选择性方面产生了优异的结果。































 京公网安备 11010802027423号
京公网安备 11010802027423号