当前位置:
X-MOL 学术
›
J. Med. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Identifying Artifacts from Large Library Docking
Journal of Medicinal Chemistry ( IF 6.8 ) Pub Date : 2024-09-10 , DOI: 10.1021/acs.jmedchem.4c01632 Yujin Wu 1 , Fangyu Liu 1 , Isabella Glenn 1 , Karla Fonseca-Valencia 1 , Lu Paris 1 , Yuyue Xiong 2 , Steven V Jerome 3 , Charles L Brooks 4 , Brian K Shoichet 1
Journal of Medicinal Chemistry ( IF 6.8 ) Pub Date : 2024-09-10 , DOI: 10.1021/acs.jmedchem.4c01632 Yujin Wu 1 , Fangyu Liu 1 , Isabella Glenn 1 , Karla Fonseca-Valencia 1 , Lu Paris 1 , Yuyue Xiong 2 , Steven V Jerome 3 , Charles L Brooks 4 , Brian K Shoichet 1
Affiliation
|
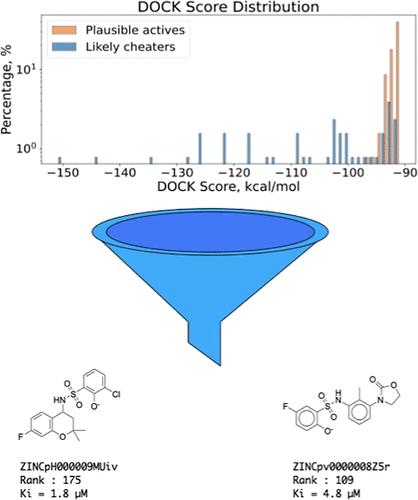
While large library docking has discovered potent ligands for multiple targets, as the libraries have grown the hit lists can become dominated by rare artifacts that cheat our scoring functions. Here, we investigate rescoring top-ranked docked molecules with orthogonal methods to identify these artifacts, exploring implicit solvent models and absolute binding free energy perturbation as cross-filters. In retrospective studies, this approach deprioritized high-ranking nonbinders for nine targets while leaving true ligands relatively unaffected. We tested the method prospectively against hits from docking against AmpC β-lactamase. We prioritized 128 high-ranking molecules for synthesis and testing, a mixture of 39 molecules flagged as likely cheaters and 89 that were plausible inhibitors. None of the predicted cheating compounds inhibited AmpC detectably, while 57% of the 89 plausible compounds did so. As our libraries continue to grow, deprioritizing docking artifacts by rescoring with orthogonal methods may find wide use.
中文翻译:

识别大型库停靠中的工件
虽然大型文库对接已经发现了针对多个靶标的有效配体,但随着文库的增长,命中列表可能会以欺骗我们评分函数的稀有伪影为主。在这里,我们研究了用正交方法对排名靠前的对接分子进行重新评分以识别这些伪影,探索隐式溶剂模型和绝对结合自由能扰动作为交叉滤波器。在回顾性研究中,这种方法降低了 9 个靶标的高秩非结合剂的优先级,而真正的配体相对不受影响。我们前瞻性地针对 AmpC β-内酰胺酶对接的命中测试了该方法。我们优先考虑了 128 个高等级分子进行合成和测试,其中 39 个分子被标记为可能的作弊分子和 89 个可能的抑制剂。预测的作弊化合物都没有检测到 AmpC 抑制,而 89 种可能的化合物中有 57% 抑制了 AmpC。随着我们的库不断增长,通过使用正交方法重新评分来降低对接伪像的优先级可能会得到广泛应用。
更新日期:2024-09-10
中文翻译:

识别大型库停靠中的工件
虽然大型文库对接已经发现了针对多个靶标的有效配体,但随着文库的增长,命中列表可能会以欺骗我们评分函数的稀有伪影为主。在这里,我们研究了用正交方法对排名靠前的对接分子进行重新评分以识别这些伪影,探索隐式溶剂模型和绝对结合自由能扰动作为交叉滤波器。在回顾性研究中,这种方法降低了 9 个靶标的高秩非结合剂的优先级,而真正的配体相对不受影响。我们前瞻性地针对 AmpC β-内酰胺酶对接的命中测试了该方法。我们优先考虑了 128 个高等级分子进行合成和测试,其中 39 个分子被标记为可能的作弊分子和 89 个可能的抑制剂。预测的作弊化合物都没有检测到 AmpC 抑制,而 89 种可能的化合物中有 57% 抑制了 AmpC。随着我们的库不断增长,通过使用正交方法重新评分来降低对接伪像的优先级可能会得到广泛应用。

































 京公网安备 11010802027423号
京公网安备 11010802027423号