当前位置:
X-MOL 学术
›
Environ. Sci. Technol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Water-Driven Surface Lattice Oxygen Activation in MnO2 for Promoted Low-Temperature NH3–SCR
Environmental Science & Technology ( IF 10.8 ) Pub Date : 2024-09-09 , DOI: 10.1021/acs.est.4c06313 Dongqi An 1, 2 , Shan Yang 3 , Qianni Cheng 1 , Wanting Yan 1 , Jingfang Sun 1 , Weixin Zou 1 , Chuanzhi Sun 3 , Changjin Tang 4 , Lin Dong 1
Environmental Science & Technology ( IF 10.8 ) Pub Date : 2024-09-09 , DOI: 10.1021/acs.est.4c06313 Dongqi An 1, 2 , Shan Yang 3 , Qianni Cheng 1 , Wanting Yan 1 , Jingfang Sun 1 , Weixin Zou 1 , Chuanzhi Sun 3 , Changjin Tang 4 , Lin Dong 1
Affiliation
|
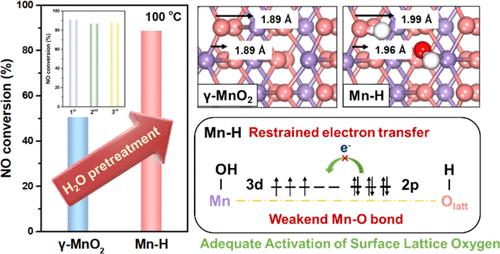
Water is ubiquitous in various heterogeneous catalytic reactions, where it can be easily adsorbed, chemically dissociated, and diffused on catalyst surfaces, inevitably influencing the catalytic process. However, the specific role of water in these reactions remains unclear. In this study, we innovatively propose that H2O-driven surface lattice oxygen activation in γ-MnO2 significantly enhances low-temperature NH3–SCR. The proton from water dissociation activates the surface lattice oxygen in γ-MnO2, giving rise to a doubling of catalytic activity (achieving 90% NO conversion at 100 °C) and remarkable stability. Comprehensive in situ characterizations and calculations reveal that spontaneous proton diffusion to the surface lattice oxygen reduces the orbital overlap between the protonated oxygen atom and its neighboring Mn atom. Consequently, the Mn–O bond is weakened and the surface lattice oxygen is effectively activated to provide excess oxygen vacancies available for converting O2 into O2–. Therefore, the redox property of Mn–H is improved, leading to enhanced NH3 oxidation-dehydrogenation and NO oxidation processes, which are crucial for low-temperature NH3–SCR. This work provides a deeper understanding and fresh perspectives on the water promotion mechanism in low-temperature NOx elimination.
中文翻译:

MnO2 中水驱表面晶格氧活化促进低温 NH3–SCR
水在各种多相催化反应中普遍存在,很容易在催化剂表面吸附、化学离解和扩散,不可避免地影响催化过程。然而,水在这些反应中的具体作用仍不清楚。在这项研究中,我们创新性地提出,H 2 O驱动的γ-MnO 2表面晶格氧活化显着增强了低温NH 3 –SCR。水离解产生的质子激活 γ-MnO 2中的表面晶格氧,使催化活性加倍(在 100 °C 时实现 90% NO 转化)和显着的稳定性。综合原位表征和计算表明,自发质子扩散到表面晶格氧减少了质子化氧原子与其相邻锰原子之间的轨道重叠。因此,Mn-O键被削弱,表面晶格氧被有效激活,提供过量的氧空位可用于将O 2转化为O 2 - 。因此,Mn-H的氧化还原性能得到改善,从而增强NH 3氧化-脱氢和NO氧化过程,这对于低温NH 3 -SCR至关重要。这项工作为低温NO x消除中的水促进机制提供了更深入的理解和新的视角。
更新日期:2024-09-09
中文翻译:

MnO2 中水驱表面晶格氧活化促进低温 NH3–SCR
水在各种多相催化反应中普遍存在,很容易在催化剂表面吸附、化学离解和扩散,不可避免地影响催化过程。然而,水在这些反应中的具体作用仍不清楚。在这项研究中,我们创新性地提出,H 2 O驱动的γ-MnO 2表面晶格氧活化显着增强了低温NH 3 –SCR。水离解产生的质子激活 γ-MnO 2中的表面晶格氧,使催化活性加倍(在 100 °C 时实现 90% NO 转化)和显着的稳定性。综合原位表征和计算表明,自发质子扩散到表面晶格氧减少了质子化氧原子与其相邻锰原子之间的轨道重叠。因此,Mn-O键被削弱,表面晶格氧被有效激活,提供过量的氧空位可用于将O 2转化为O 2 - 。因此,Mn-H的氧化还原性能得到改善,从而增强NH 3氧化-脱氢和NO氧化过程,这对于低温NH 3 -SCR至关重要。这项工作为低温NO x消除中的水促进机制提供了更深入的理解和新的视角。

































 京公网安备 11010802027423号
京公网安备 11010802027423号