当前位置:
X-MOL 学术
›
Cell Stem Cell
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Modeling the atrioventricular conduction axis using human pluripotent stem cell-derived cardiac assembloids
Cell Stem Cell ( IF 19.8 ) Pub Date : 2024-09-10 , DOI: 10.1016/j.stem.2024.08.008 Jiuru Li 1 , Alexandra Wiesinger 1 , Lianne Fokkert 1 , Priscilla Bakker 1 , Dylan K de Vries 2 , Anke J Tijsen 2 , Yigal M Pinto 3 , Arie O Verkerk 4 , Vincent M Christoffels 1 , Gerard J J Boink 5 , Harsha D Devalla 1
Cell Stem Cell ( IF 19.8 ) Pub Date : 2024-09-10 , DOI: 10.1016/j.stem.2024.08.008 Jiuru Li 1 , Alexandra Wiesinger 1 , Lianne Fokkert 1 , Priscilla Bakker 1 , Dylan K de Vries 2 , Anke J Tijsen 2 , Yigal M Pinto 3 , Arie O Verkerk 4 , Vincent M Christoffels 1 , Gerard J J Boink 5 , Harsha D Devalla 1
Affiliation
|
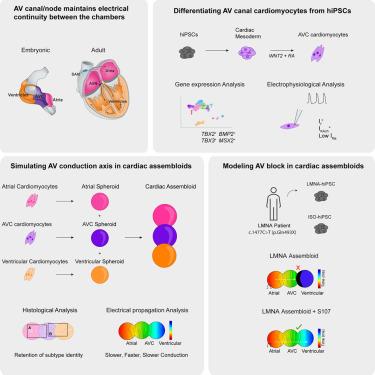
The atrioventricular (AV) conduction axis provides electrical continuity between the atrial and ventricular chambers. The “nodal” cardiomyocytes populating this region (AV canal in the embryo, AV node from fetal stages onward) propagate impulses slowly, ensuring sequential contraction of the chambers. Dysfunction of AV nodal tissue causes severe disturbances in rhythm and contraction, and human models that capture its salient features are limited. Here, we report an approach for the reproducible generation of AV canal cardiomyocytes (AVCMs) with in vivo-like gene expression and electrophysiological profiles. We created the so-called “assembloids” composed of atrial, AVCM, and ventricular spheroids, which effectively recapitulated unidirectional conduction and the “fast-slow-fast” activation pattern typical for the vertebrate heart. We utilized these systems to reveal intracellular calcium mishandling as the basis of LMNA-associated AV conduction block. In sum, our study introduces novel cell differentiation and tissue construction strategies to facilitate the study of complex disorders affecting heart rhythm.
中文翻译:

使用人类多能干细胞来源的心脏组件对房室传导轴进行建模
房室 (AV) 传导轴在心房和心室之间提供电连续性。填充该区域的“淋巴结”心肌细胞(胚胎中的 AV 管,从胎儿阶段开始的 AV 结)缓慢传播冲动,确保腔室的顺序收缩。房室结组织功能障碍会导致节律和收缩的严重紊乱,捕捉其显着特征的人体模型是有限的。在这里,我们报道了一种可重复生成具有 体内样基因表达和电生理特征的 AV 管心肌细胞 (AVCMs) 的方法。我们创建了由心房、AVCM 和心室球状球组成的所谓“组合体”,它有效地概括了脊椎动物心脏典型的单向传导和“快-慢-快”激活模式。我们利用这些系统揭示了细胞内钙处理不当是 LMNA 相关 AV 传导阻滞的基础。总之,我们的研究引入了新的细胞分化和组织构建策略,以促进影响心律的复杂疾病的研究。
更新日期:2024-09-10
中文翻译:

使用人类多能干细胞来源的心脏组件对房室传导轴进行建模
房室 (AV) 传导轴在心房和心室之间提供电连续性。填充该区域的“淋巴结”心肌细胞(胚胎中的 AV 管,从胎儿阶段开始的 AV 结)缓慢传播冲动,确保腔室的顺序收缩。房室结组织功能障碍会导致节律和收缩的严重紊乱,捕捉其显着特征的人体模型是有限的。在这里,我们报道了一种可重复生成具有 体内样基因表达和电生理特征的 AV 管心肌细胞 (AVCMs) 的方法。我们创建了由心房、AVCM 和心室球状球组成的所谓“组合体”,它有效地概括了脊椎动物心脏典型的单向传导和“快-慢-快”激活模式。我们利用这些系统揭示了细胞内钙处理不当是 LMNA 相关 AV 传导阻滞的基础。总之,我们的研究引入了新的细胞分化和组织构建策略,以促进影响心律的复杂疾病的研究。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号