当前位置:
X-MOL 学术
›
J. Comput. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Pyrolytic conversion of glucose into hydroxymethylfurfural and furfural: Benchmark quantum-chemical calculations
Journal of Computational Chemistry ( IF 3.4 ) Pub Date : 2024-09-09 , DOI: 10.1002/jcc.27503 Roberto López 1 , Dimas Suárez 2
Journal of Computational Chemistry ( IF 3.4 ) Pub Date : 2024-09-09 , DOI: 10.1002/jcc.27503 Roberto López 1 , Dimas Suárez 2
Affiliation
|
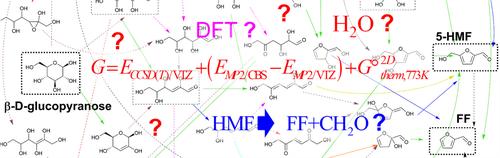
Quantum chemical methods have been intensively applied to study the pyrolytic conversion of glucose into hydroxymethylfurfural (HMF) and furfural (FF). Herein, we collect the most relevant mechanistic proposals from the recent literature and organize them into a single reaction network. All the transition structures (TSs) and intermediates are characterized using highly accurate ab initio methods and the possible reaction pathways are assessed in terms of the Gibbs energies of the TSs and intermediates with respect to β-glucopyranose, selecting a 2D ideal-gas standard state at 773 K to represent the pyrolysis conditions. Several pathways can lead to the formation of both HMF and FF passing through rate-determining TSs that have ΔG‡ values of ~49–50 kcal/mol. Both water-assisted mechanisms and nonspecific environmental effects have a minor impact on the Gibbs energy profiles. We find that the HMF → FF + CH2O fragmentation has a small ΔrxnG value and an accessible ΔG‡ barrier. Our computational results, which are in consonance with the kinetic parameters derived from lumped models, the results of isotopic labeling experiments and the reported HMF/FF molecular ratios, could be useful for modeling studies including on nonequilibrium kinetic effects that may render more information about product yields and the relevance of the various pathways.
中文翻译:

葡萄糖热解转化为羟甲基糠醛和糠醛:基准量子化学计算
量子化学方法已被广泛应用于研究葡萄糖向羟甲基糠醛 (HMF) 和糠醛 (FF) 的热解转化。在这里,我们从最近的文献中收集了最相关的机理建议,并将它们组织成一个单一的反应网络。所有过渡结构 (TS) 和中间体都使用高精度的从头计算方法进行表征,并根据 TS 和中间体相对于 β-吡喃葡萄糖的吉布斯能评估可能的反应途径,选择 773 K 的 2D 理想气体标准态来表示热解条件。有几种途径可导致 HMF 和 FF 的形成,通过具有 ΔG‡ 值为 ~49–50 kcal/mol 的速率决定 TS。水辅助机制和非特异性环境效应对吉布斯能量剖面的影响很小。我们发现 HMF → FF + CH2O 碎裂具有较小的 ΔrxnG 值和可接近的 ΔG‡ 屏障。我们的计算结果与从集总模型得出的动力学参数、同位素标记实验的结果和报告的 HMF/FF 分子比一致,可用于建模研究,包括非平衡动力学效应,这可能会提供有关产品产量和各种途径相关性的更多信息。
更新日期:2024-09-09
中文翻译:

葡萄糖热解转化为羟甲基糠醛和糠醛:基准量子化学计算
量子化学方法已被广泛应用于研究葡萄糖向羟甲基糠醛 (HMF) 和糠醛 (FF) 的热解转化。在这里,我们从最近的文献中收集了最相关的机理建议,并将它们组织成一个单一的反应网络。所有过渡结构 (TS) 和中间体都使用高精度的从头计算方法进行表征,并根据 TS 和中间体相对于 β-吡喃葡萄糖的吉布斯能评估可能的反应途径,选择 773 K 的 2D 理想气体标准态来表示热解条件。有几种途径可导致 HMF 和 FF 的形成,通过具有 ΔG‡ 值为 ~49–50 kcal/mol 的速率决定 TS。水辅助机制和非特异性环境效应对吉布斯能量剖面的影响很小。我们发现 HMF → FF + CH2O 碎裂具有较小的 ΔrxnG 值和可接近的 ΔG‡ 屏障。我们的计算结果与从集总模型得出的动力学参数、同位素标记实验的结果和报告的 HMF/FF 分子比一致,可用于建模研究,包括非平衡动力学效应,这可能会提供有关产品产量和各种途径相关性的更多信息。































 京公网安备 11010802027423号
京公网安备 11010802027423号