American Journal of Hematology ( IF 10.1 ) Pub Date : 2024-09-10 , DOI: 10.1002/ajh.27475 Talha Badar 1 , Ravi Narra 2 , Alice S Mims 3 , Michael G Heckman 4 , Rory M Shallis 5 , Sheikh Fahad 4 , Cameron Hunter 5 , Vamsi Kota 6 , Tamer Adel Othman 7 , Brian Jonas 7 , Shreya Desai 6 , Guilherme Sacchi de Camargo Correia 1 , Anand Patel 8 , Adam S DuVall 8 , Neil Palmisiano 9 , Emily Curran 10 , Zulfa Omer 10 , Anjali Advani 11 , Ehab Atallah 2 , Mark Litzow 12
|
Prior to the advent of BCR::ABL1-directed tyrosine kinase inhibitors (TKI), the long-term survival of patients with Philadelphia chromosome-positive (Ph+) acute lymphoblastic leukemia (ALL) was dismal in the range of 5%–15%.1 The introduction of TKI in the treatment regimens has significantly improved disease outcomes.2 Historically, allogeneic stem cell transplantation (allo-HCT) was considered the consolidation of choice for patients with Ph+ ALL in first complete remission (CR) given its association with improved long-term survival, which has not changed in the post TKI era.3 Recently, it was reported that the survival benefit of consolidation allo-HCT in patients who achieve a BCR::ABL1 transcript level of <0.01% via reverse-transcription PCR-based quantitative assay (RT-PCR) by 90 days post induction may be questionable.4 In this study, utilizing multi-institutional real-world data, we analyzed the outcomes of patients with Ph+ ALL with evolving frontline therapies and the significance of allo-HCT in improving long-term outcome.
We conducted a real-world, multi-institutional analysis through the COMMAND (Consortium on Myeloid Malignancies and Neoplastic Diseases). The study was conducted after obtaining approval from the Institutional Review Board (IRB), adhering to the ethical standards of the Declaration of Helsinki. A total of 431 adult (≥ 18 years) patients with Ph+ ALL diagnosed between May 2003 and December 2022 were evaluated to assess for response and survival with intensive (IC) and nonintensive (NIC) frontline TKI-based treatment combinations. We also assessed the survival benefit of allo-HCT including patients who achieved complete molecular remission (CMR) at 3 months from the time of induction therapy. Details on methods and statistical analysis performed are available in Supplementary Material.
The median age of patients was 52 years (range [R] 19–85), and 50% were male (Supplementary Table 1). The most common BCR::ABL1 breakpoint was the p190 fusion protein (51%), 55% of patients had additional cytogenetic (CG) abnormalities apart from t (9;22), and 35 (8%) patients had CNS disease at diagnosis. Most patients received TKI in combination with an IC (n = 317 [74%]), with 26% (n = 112) receiving TKI with an NIC; data are not available on two patients. During induction, most patients received a second-generation TKI (70% [n = 298]); 87 patients (20%) received imatinib and 41 patients (10%) received ponatinib. A total of 205 of 431 (47.5%) patients received allo-HCT in CR1. The complete remission (CR/CRi) rate was 95% (n = 412 of 431 were evaluable for response). Fifty-one percent of patients achieved MRD negativity by multiparameter flow cytometry (MFC) after induction and 67% (N = 335 evaluable) had CMR by RT-PCR at 3 months. MRD negativity rate with IC, NIC, and successive generation of TKI are summarized in Supplementary Table 1.
With a median follow-up after diagnosis of 2.7 years (R, 0.02–17.3 years), the median RFS of the entire cohort was 122.8 months: 129 months and 36.93 months with IC + TKI and NIC + TKI, respectively (p = .003; Supplementary Figure 1A). The 3-year RFS based on TKI used during induction was 47.5% and 66%, with first-generation and second-/third-generation TKI, respectively (hazard ratio [HR] 0.76, p = .087; Supplementary Figure 1B).
The median OS of the entire cohort was 112.4 months; 123.1 and 49.40 months when evaluating patients receiving IC + TKI and NIC+ TKI, respectively (p = .01; Supplementary Figure 1C). The 3-year OS based on TKI used during induction was 55.7% and 71.1%, with first-generation and second-/third-generation TKI, respectively (HR 0.66, p = .026; Supplementary Figure 1D).
In multivariable analysis for RFS after adjusting for potential confounding variables, significant associations were observed with age at diagnosis (HR [age >61 vs. ≤40 years]; 2.01, p = <.001), additional CG abnormalities (HR; 1.35, p = .03), type of TKI during induction (HR [dasatinib/nilotinib or ponatinib vs. imatinib]; 0.70, p = .03). Allo-HCT showed favorable impact on RFS irrespective of patients achieving CMR (HR; 0.32, p = <.001) or not achieving CMR at 3 months (HR; 0.22, p = <.001) (Supplementary Table 2). Next, we evaluated the association of MFC-MRD negativity and CMR at 3 months with RFS and did not find any significance either in unadjusted analysis (HR 0.93, p = .59/HR 0.77, p = .06) or in multivariable analysis (HR 1.03, p = .85/HR 0.89, p = .44), respectively (Supplementary Table 3, Supplementary Figure 2A,B).
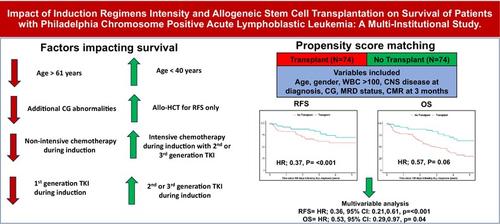
To avoid immortal-time bias, we displayed outcomes after a baseline time point of 180 days following diagnosis, where the allo-HCT and non-allo-HCT group did not have an event (relapse or death) in 180 days following diagnosis and observed significantly better RFS with allo-HCT (HR; 0.39, p = <.001; Figure 1A), and the RFS was consistently favorable post 180 days in allo-HCT group utilizing propensity-score-matched cohort (HR; 0.37, p = <.001) (Figure 1B).

After adjusting for variables, highlighted in Supplementary Table 4, we compared outcomes between propensity-score-matched allo-HCT and non-allo-HCT patients. Relapse-free survival was better for allo-HCT patients compared with non-allo-HCT patients in both unadjusted analysis (HR; 0.37, p = <.001, Figure 1C) and in multivariable analysis (HR; 0.36, p = <.001), Supplementary Table 5.
In multivariable analysis for OS, significant associations were observed with age at diagnosis (HR [age >61 vs. ≤40 years]; 2.89, p < .001) and type of TKI use during induction (HR [2nd/3rd vs. 1st generation]; 0.66, p = .02), Supplementary Table 6. Similarly, no significant association was found between OS with MFC-MRD negativity (HR 1.12, p = .56, Supplementary Figure 2C). While we observe OS benefit with CMR in unadjusted analysis (Supplementary Figure 2D), it was not retained in multivariable analysis (HR 0.90, p = .55) (Supplementary Table 3). Allo-HCT did not show significant impact on OS based on achieving CMR (HR; 0.87, p = .59) or not achieving CMR (HR; 0.48, p = .11) at 3 months. We did not observe significant improvement in OS with allo-HCT at 180-day time point (HR; 0.75, p = .09, Figure 1C), or after utilizing propensity match score (HR; 0.57, p = .06 (Figure 1D) in unadjusted analysis. In multivariable analysis, allo-HCT showed significance using propensity match score (HR = 0.53, p = .04), Supplementary Table 5.
In our multicenter real-world analysis, after adjusting for confounding variables, we observed significantly inferior RFS and OS for older age at diagnosis (>61 vs. ≤ 40 years), and use of first-generation vs. second-/third-generation TKI during induction. In multivariable analysis, the regimen intensity (NIC vs. IC) did not appear to retain significance for RFS or OS. Allogeneic stem cell transplantation was beneficial in improving RFS but not OS in patients who achieve CMR at 3 months.
The availability and incorporation of BCR::ABL1-directed TKI into the frontline regimens has improved the survival of patients with Ph+ ALL. While all TKI combinations with chemotherapy have shown to improve survival compared with chemotherapy alone, second- or third-generation TKI have shown to benefit the most with longer OS and lesser emergence of resistant mutations.2 Ponatinib is among the most potent BCR::ABL1-directed TKIs, with effectiveness in wild-type as well mutated ABL1, especially T315I mutation. Recently, a phase III study evaluating ponatinib versus imatinib with NIC in Ph+ ALL showed superior MRD-negative CR rates and trend toward better event-free survival, favoring ponatinib.5 In our analysis and in line with previous observations, we observed significant improvement in RFS and OS with second- or third-generation TKI-based frontline treatment combinations compared with imatinib, and we conclude that potent TKI combinations should be used upfront in Ph+ ALL for best long-term outcomes.
Intensive chemotherapy induction combination with a BCR::ABL1-directed TKI can be challenging in elderly patients with Ph+ ALL. Several prospective studies with NIC plus TKI combination have showed favorable CR rates in the range of 90%–95%; however, long-term survival remains modest in the range of 35%–45%.1 Similarly, we have observed significantly inferior survival with NIC + TKI compared with IC + TKI combinations. However, chemotherapy-free induction using blinatumomab + TKI upfront is gaining attraction due to better tolerability and efficacy,6 and it is being evaluated in randomized fashion with IC + TKI combination.
With the evolution of novel and extremely effective treatment combinations for the management of Ph+ ALL, the role of allo-HCT in improving OS is being examined. In recently conducted retrospective analysis, 230 patients with Ph+ ALL who attained CMR at 90 days from diagnosis were analyzed to study whether allo-HCT was effective in improving OS.4 The study revealed that allo-HCT was effective in reducing the cumulative incidence of relapse but did not have an impact on improving OS. Similar to these observations, we did observe favorable impact of allo-HCT on improving RFS regardless of achieving or not achieving CMR at 3 months but not so for OS. However, after propensity score matching and multivariable analysis, we did see benefit of allo-HCT in improving RFS and OS. We acknowledge the limitation of our analysis, heterogeneity in data, the sample size of the propensity-score-matched group was relatively small, and therefore the possibility of a type II error (i.e., a false-negative finding) is important to consider. Prospective, randomized trials are warranted to better understand who will likely to derive survival benefit from allo-HCT in Ph+ ALL.
While our study in a large cohort of patients provides a real-world perspective on treatment outcomes of Ph+ ALL patients with evolving frontline therapies, it is subject to the limitations of any retrospective analysis and the heterogeneity in data collected.































 京公网安备 11010802027423号
京公网安备 11010802027423号