当前位置:
X-MOL 学术
›
Phys. Chem. Chem. Phys.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
How lithium-ion batteries work conceptually: thermodynamics of Li bonding in idealized electrodes
Physical Chemistry Chemical Physics ( IF 2.9 ) Pub Date : 2024-09-10 , DOI: 10.1039/d4cp00818a
Sam H Finkelstein 1 , Marco Ricci 2, 3 , Tom Bötticher 4 , Klaus Schmidt-Rohr 1
Physical Chemistry Chemical Physics ( IF 2.9 ) Pub Date : 2024-09-10 , DOI: 10.1039/d4cp00818a
Sam H Finkelstein 1 , Marco Ricci 2, 3 , Tom Bötticher 4 , Klaus Schmidt-Rohr 1
Affiliation
|
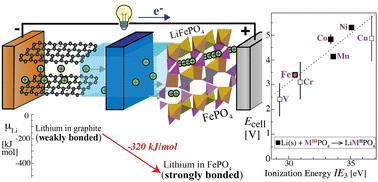
A good explanation of lithium-ion batteries (LIBs) needs to convincingly account for the spontaneous, energy-releasing movement of lithium ions and electrons out of the negative and into the positive electrode, the defining characteristic of working LIBs. We analyze a discharging battery with a two-phase LiFePO4/FePO4 positive electrode (cathode) from a thermodynamic perspective and show that, compared to loosely-bound lithium in the negative electrode (anode), lithium in the ionic positive electrode is more strongly bonded, moves there in an energetically downhill irreversible process, and ends up trapped in the positive electrode. Only a sufficiently high charging voltage can drive it back to the other electrode. Since the stronger bonding in the positive electrode lowers the energy by ∼320 kJ mol−1, a lot of energy is released. This explanation is quantitatively supported by an analysis of cohesive-energy differences of the electrode materials. Since electrons are only intermediates in the discharge reaction and the chemical potential of the electron cannot be measured, electrons do not need to be assigned a distinct energetic role. The incorporation of Li+ and an electron into the cathode is accompanied by the reduction of another ion or atom, usually a transition metal such as Fe or Co. The metal's ionization energy in the corresponding oxidation step correlates with the cell voltage, based on a decomposition of cohesive energy into electronic and ionic components. We relate the differences in cohesive energies to the chemical potential of lithium atoms, which is quantified, for instance for a two-phase electrode. The analysis is extended to a single-phase LixCoO2 cathode, whose average voltage can be calculated from the cohesive-energy difference between LiCoO2 and CoO2.
中文翻译:

锂离子电池的工作原理:理想电极中锂键合的热力学
对锂离子电池 (LIB) 的良好解释需要令人信服地解释锂离子和电子从负极进入正极的自发能量释放运动,这是工作锂离子电池的定义特征。我们从热力学角度分析了具有两相LiFePO 4 /FePO 4正极(正极)的放电电池,结果表明,与负极(负极)中松散结合的锂相比,离子正极中的锂含量更高。牢固地结合在一起,以能量下坡的不可逆过程移动到那里,并最终被困在正极中。只有足够高的充电电压才能将其驱动回另一个电极。由于正极中更强的键合使能量降低了约320 kJ mol -1 ,因此释放了大量能量。这种解释得到了电极材料内聚能差异分析的定量支持。由于电子只是放电反应中的中间体,并且电子的化学势无法测量,因此不需要为电子分配独特的能量角色。 Li +和电子进入阴极的过程伴随着另一个离子或原子的还原,通常是过渡金属,如 Fe 或 Co。金属在相应氧化步骤中的电离能与电池电压相关,基于将内聚能分解为电子和离子成分。我们将内聚能的差异与锂原子的化学势联系起来,例如对于两相电极,这是量化的。 该分析扩展到单相Li x CoO 2阴极,其平均电压可以根据LiCoO 2和CoO 2之间的内聚能差计算。
更新日期:2024-09-10
中文翻译:

锂离子电池的工作原理:理想电极中锂键合的热力学
对锂离子电池 (LIB) 的良好解释需要令人信服地解释锂离子和电子从负极进入正极的自发能量释放运动,这是工作锂离子电池的定义特征。我们从热力学角度分析了具有两相LiFePO 4 /FePO 4正极(正极)的放电电池,结果表明,与负极(负极)中松散结合的锂相比,离子正极中的锂含量更高。牢固地结合在一起,以能量下坡的不可逆过程移动到那里,并最终被困在正极中。只有足够高的充电电压才能将其驱动回另一个电极。由于正极中更强的键合使能量降低了约320 kJ mol -1 ,因此释放了大量能量。这种解释得到了电极材料内聚能差异分析的定量支持。由于电子只是放电反应中的中间体,并且电子的化学势无法测量,因此不需要为电子分配独特的能量角色。 Li +和电子进入阴极的过程伴随着另一个离子或原子的还原,通常是过渡金属,如 Fe 或 Co。金属在相应氧化步骤中的电离能与电池电压相关,基于将内聚能分解为电子和离子成分。我们将内聚能的差异与锂原子的化学势联系起来,例如对于两相电极,这是量化的。 该分析扩展到单相Li x CoO 2阴极,其平均电压可以根据LiCoO 2和CoO 2之间的内聚能差计算。



































 京公网安备 11010802027423号
京公网安备 11010802027423号