当前位置:
X-MOL 学术
›
Phys. Chem. Chem. Phys.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Synthesis, crystal structure analysis, computational modelling and evaluation of anti-cervical cancer activity of novel 1,5-dicyclooctyl thiocarbohydrazone
Physical Chemistry Chemical Physics ( IF 2.9 ) Pub Date : 2024-09-10 , DOI: 10.1039/d4cp02286f Soni Shukla 1 , Prince Trivedi 1 , Delna Johnson 2 , Pulkit Sharma 1 , Abhinav Jha 1 , Habiba Khan 3 , Vijay Thiruvenkatam 2 , Monisha Banerjee 3 , Abha Bishnoi 1
Physical Chemistry Chemical Physics ( IF 2.9 ) Pub Date : 2024-09-10 , DOI: 10.1039/d4cp02286f Soni Shukla 1 , Prince Trivedi 1 , Delna Johnson 2 , Pulkit Sharma 1 , Abhinav Jha 1 , Habiba Khan 3 , Vijay Thiruvenkatam 2 , Monisha Banerjee 3 , Abha Bishnoi 1
Affiliation
|
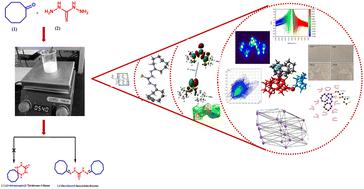
Thiocarbazones are widely used as bioactive and pharmaceutical intermediates in medicinal chemistry and have been shown to exhibit diverse biological and pharmacological activities such as antimicrobial, anticancer, anti-viral, anti-convulsant and anti-inflammatory etc. In continuation of our interest in biologically active heterocycles and in an attempt to synthesize a spiro derivative, 1,2,4,5-tetraazaspiro[5.7]tridecane-3-thione, herein, the synthesis of 1,5-dicyclooctyl thiocarbohydrazone (3) has been reported via reaction of the cyclooctanone and thiocarbohydrazide. The structure was assigned on the basis of detailed spectral analysis and also confirmed by X-ray crystal studies. The Hirshfeld surface analysis indicates that the most significant interaction is S⋯H (12.7%). The presentation of mechanistic aspects regarding the plausible route of its formation has also been included. The first hyperpolarizability (β0) was found to be 10.22 × 10−30 esu, which indicates that the compound exhibits good non-linear optical properties. The density functional theory (DFT) method has been used to characterize the spectroscopic properties and vibrational analysis of 1,5-dicyclooctyl thiocarbohydrazone (3) theoretically. The compound and cisplatin (standard) were screened for their antiproliferative activity against the human cervical cancer cell line (SiHa) and they exhibited significant activity with IC50 values of 250 μM and 15 μM, respectively. The inhibitory nature of the title compound against viral oncoprotein E6 was confirmed by studies using molecular docking analysis. The results of biological activity and in silico analysis indicate that the synthesized molecule could act as a precursor for the synthesis of new heterocyclic derivatives of medicinal importance.
中文翻译:

新型1,5-二环辛基硫卡腙的合成、晶体结构分析、计算建模及抗宫颈癌活性评价
硫卡巴腙在药物化学中被广泛用作生物活性和药物中间体,并已被证明具有多种生物和药理活性,如抗菌、抗癌、抗病毒、抗惊厥和抗炎等。杂环并试图合成螺衍生物 1,2,4,5-四氮杂螺[5.7]十三烷-3-硫酮,本文报道了通过以下反应合成了 1,5-二环辛基硫代碳腙 ( 3 )环辛酮和硫代碳酰肼。该结构是根据详细的光谱分析确定的,并通过 X 射线晶体研究证实。 Hirshfeld 表面分析表明最显着的相互作用是 S⋯H (12.7%)。还包括有关其形成的合理途径的机械方面的介绍。第一超极化率( β 0 )为10.22 × 10 -30 esu,这表明该化合物表现出良好的非线性光学性质。采用密度泛函理论(DFT)方法从理论上表征了1,5-二环辛基硫卡腙( 3 )的光谱性质和振动分析。筛选了该化合物和顺铂(标准品)对人宫颈癌细胞系(SiHa)的抗增殖活性,它们表现出显着的活性,IC 50值分别为250 μM和15 μM。使用分子对接分析的研究证实了标题化合物对病毒癌蛋白 E6 的抑制性质。 生物活性和计算机分析结果表明,合成的分子可以作为合成具有药用价值的新型杂环衍生物的前体。
更新日期:2024-09-10
中文翻译:

新型1,5-二环辛基硫卡腙的合成、晶体结构分析、计算建模及抗宫颈癌活性评价
硫卡巴腙在药物化学中被广泛用作生物活性和药物中间体,并已被证明具有多种生物和药理活性,如抗菌、抗癌、抗病毒、抗惊厥和抗炎等。杂环并试图合成螺衍生物 1,2,4,5-四氮杂螺[5.7]十三烷-3-硫酮,本文报道了通过以下反应合成了 1,5-二环辛基硫代碳腙 ( 3 )环辛酮和硫代碳酰肼。该结构是根据详细的光谱分析确定的,并通过 X 射线晶体研究证实。 Hirshfeld 表面分析表明最显着的相互作用是 S⋯H (12.7%)。还包括有关其形成的合理途径的机械方面的介绍。第一超极化率( β 0 )为10.22 × 10 -30 esu,这表明该化合物表现出良好的非线性光学性质。采用密度泛函理论(DFT)方法从理论上表征了1,5-二环辛基硫卡腙( 3 )的光谱性质和振动分析。筛选了该化合物和顺铂(标准品)对人宫颈癌细胞系(SiHa)的抗增殖活性,它们表现出显着的活性,IC 50值分别为250 μM和15 μM。使用分子对接分析的研究证实了标题化合物对病毒癌蛋白 E6 的抑制性质。 生物活性和计算机分析结果表明,合成的分子可以作为合成具有药用价值的新型杂环衍生物的前体。































 京公网安备 11010802027423号
京公网安备 11010802027423号