当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Copper-Catalyzed Nucleophilic Cycloisomerization Cascade Constructing Azepinoindolizine
Organic Letters ( IF 4.9 ) Pub Date : 2024-09-09 , DOI: 10.1021/acs.orglett.4c02933 Jingpeng Han 1 , Xuan Tang 2 , Xue Cheng 2 , Tu Zeng 2 , Yi Tian 2 , Yingjian Gong 2 , Baosheng Li 2
Organic Letters ( IF 4.9 ) Pub Date : 2024-09-09 , DOI: 10.1021/acs.orglett.4c02933 Jingpeng Han 1 , Xuan Tang 2 , Xue Cheng 2 , Tu Zeng 2 , Yi Tian 2 , Yingjian Gong 2 , Baosheng Li 2
Affiliation
|
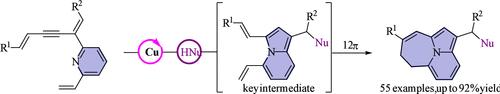
Numerous effective bioisosteric replacements have been identified through substituting scaffolds and functional groups in lead molecules with alternative ones that preserve or enhance the desired biological activity of the original compound. Here, a copper-catalyzed nucleophilic cycloisomerization was developed to access potential bioisosteric replacements of azepinoindole. In this process, “tetra-alkene” characteristic of indolizine undergoes a 12π electrocyclization, offering a complementary method to obtain azepinoindolizine derivatives that are otherwise challenging to prepare through conventional means.
中文翻译:

铜催化亲核环异构化级联构建氮杂吲哚嗪
通过用保留或增强原始化合物所需生物活性的替代物替代先导分子中的支架和官能团,已经鉴定出许多有效的生物电子等排替代物。在这里,开发了铜催化的亲核环异构化以获得氮杂吲哚的潜在生物等排替代品。在此过程中,中氮茚的“四烯”特征经历了12π电环化,为获得氮杂中氮茚衍生物提供了一种补充方法,而传统方法很难制备这些衍生物。
更新日期:2024-09-09
中文翻译:

铜催化亲核环异构化级联构建氮杂吲哚嗪
通过用保留或增强原始化合物所需生物活性的替代物替代先导分子中的支架和官能团,已经鉴定出许多有效的生物电子等排替代物。在这里,开发了铜催化的亲核环异构化以获得氮杂吲哚的潜在生物等排替代品。在此过程中,中氮茚的“四烯”特征经历了12π电环化,为获得氮杂中氮茚衍生物提供了一种补充方法,而传统方法很难制备这些衍生物。































 京公网安备 11010802027423号
京公网安备 11010802027423号