当前位置:
X-MOL 学术
›
Mol. Microbiol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Cytosolic Factors Controlling PASTA Kinase-Dependent ReoM Phosphorylation
Molecular Microbiology ( IF 2.6 ) Pub Date : 2024-09-08 , DOI: 10.1111/mmi.15307 Patricia Rothe 1 , Sabrina Wamp 1 , Lisa Rosemeyer 1 , Jeanine Rismondo 2 , Joerg Doellinger 3 , Angelika Gründling 2 , Sven Halbedel 1, 4
Molecular Microbiology ( IF 2.6 ) Pub Date : 2024-09-08 , DOI: 10.1111/mmi.15307 Patricia Rothe 1 , Sabrina Wamp 1 , Lisa Rosemeyer 1 , Jeanine Rismondo 2 , Joerg Doellinger 3 , Angelika Gründling 2 , Sven Halbedel 1, 4
Affiliation
|
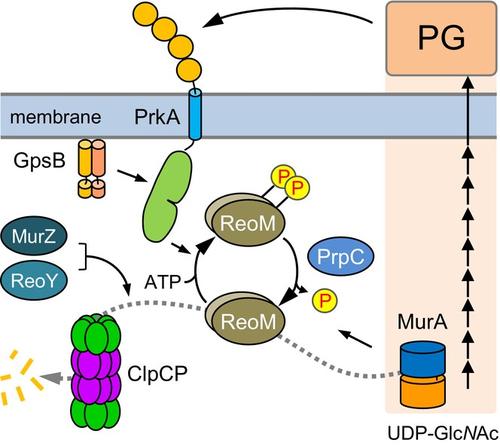
Bacteria adapt the biosynthesis of their envelopes to specific growth conditions and prevailing stress factors. Peptidoglycan (PG) is the major component of the cell wall in Gram-positive bacteria, where PASTA kinases play a central role in PG biosynthesis regulation. Despite their importance for growth, cell division and antibiotic resistance, the mechanisms of PASTA kinase activation are not fully understood. ReoM, a recently discovered cytosolic phosphoprotein, is one of the main substrates of the PASTA kinase PrkA in the Gram-positive human pathogen Listeria monocytogenes. Depending on its phosphorylation, ReoM controls proteolytic stability of MurA, the first enzyme in the PG biosynthesis pathway. The late cell division protein GpsB has been implicated in PASTA kinase signalling. Consistently, we show that L. monocytogenes prkA and gpsB mutants phenocopied each other. Analysis of in vivo ReoM phosphorylation confirmed GpsB as an activator of PrkA leading to the description of structural features in GpsB that are important for kinase activation. We further show that ReoM phosphorylation is growth phase-dependent and that this kinetic is reliant on the protein phosphatase PrpC. ReoM phosphorylation was inhibited in mutants with defects in MurA degradation, leading to the discovery that MurA overexpression prevented ReoM phosphorylation. Overexpressed MurA must be able to bind its substrates and interact with ReoM to exert this effect, but the extracellular PASTA domains of PrkA or MurJ flippases were not required. Our results indicate that intracellular signals control ReoM phosphorylation and extend current models describing the mechanisms of PASTA kinase activation.
中文翻译:

控制 PASTA 激酶依赖性 ReoM 磷酸化的胞质因子
细菌根据特定的生长条件和主要的胁迫因素调整其包膜的生物合成。肽聚糖 (PG) 是革兰氏阳性菌细胞壁的主要成分,其中 PASTA 激酶在 PG 生物合成调节中起着核心作用。尽管它们对生长、细胞分裂和抗生素耐药性很重要,但 PASTA 激酶激活的机制尚不完全清楚。ReoM 是一种最近发现的胞质磷蛋白,是革兰氏阳性人病原体单核细胞增生李斯特菌中 PASTA 激酶 PrkA 的主要底物之一。根据其磷酸化,ReoM 控制 MurA 的蛋白水解稳定性,MurA 是 PG 生物合成途径中的第一种酶。晚期细胞分裂蛋白 GpsB 与 PASTA 激酶信号转导有关。一致地,我们表明单核细胞增生李斯特菌 prkA 和 gpsB 突变体相互表型复制。体内 ReoM 磷酸化分析证实 GpsB 是 PrkA 的激活剂,导致描述了 GpsB 中对激酶激活很重要的结构特征。我们进一步表明 ReoM 磷酸化是生长阶段依赖性的,并且这种动力学依赖于蛋白质磷酸酶 PrpC。ReoM 磷酸化在 MurA 降解缺陷的突变体中受到抑制,导致发现 MurA 过表达阻止了 ReoM 磷酸化。过表达的 MurA 必须能够结合其底物并与 ReoM 相互作用才能发挥这种作用,但不需要 PrkA 或 MurJ 翻转酶的细胞外 PASTA 结构域。我们的结果表明,细胞内信号控制 ReoM 磷酸化并扩展了当前描述 PASTA 激酶激活机制的模型。
更新日期:2024-09-08
中文翻译:

控制 PASTA 激酶依赖性 ReoM 磷酸化的胞质因子
细菌根据特定的生长条件和主要的胁迫因素调整其包膜的生物合成。肽聚糖 (PG) 是革兰氏阳性菌细胞壁的主要成分,其中 PASTA 激酶在 PG 生物合成调节中起着核心作用。尽管它们对生长、细胞分裂和抗生素耐药性很重要,但 PASTA 激酶激活的机制尚不完全清楚。ReoM 是一种最近发现的胞质磷蛋白,是革兰氏阳性人病原体单核细胞增生李斯特菌中 PASTA 激酶 PrkA 的主要底物之一。根据其磷酸化,ReoM 控制 MurA 的蛋白水解稳定性,MurA 是 PG 生物合成途径中的第一种酶。晚期细胞分裂蛋白 GpsB 与 PASTA 激酶信号转导有关。一致地,我们表明单核细胞增生李斯特菌 prkA 和 gpsB 突变体相互表型复制。体内 ReoM 磷酸化分析证实 GpsB 是 PrkA 的激活剂,导致描述了 GpsB 中对激酶激活很重要的结构特征。我们进一步表明 ReoM 磷酸化是生长阶段依赖性的,并且这种动力学依赖于蛋白质磷酸酶 PrpC。ReoM 磷酸化在 MurA 降解缺陷的突变体中受到抑制,导致发现 MurA 过表达阻止了 ReoM 磷酸化。过表达的 MurA 必须能够结合其底物并与 ReoM 相互作用才能发挥这种作用,但不需要 PrkA 或 MurJ 翻转酶的细胞外 PASTA 结构域。我们的结果表明,细胞内信号控制 ReoM 磷酸化并扩展了当前描述 PASTA 激酶激活机制的模型。






























 京公网安备 11010802027423号
京公网安备 11010802027423号