当前位置:
X-MOL 学术
›
Eur. J. Med. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Design and synthesis of pyrano[2,3-c]pyrazole-4-aminoquinoline hybrids as effective antimalarial compounds
European Journal of Medicinal Chemistry ( IF 6.0 ) Pub Date : 2024-09-05 , DOI: 10.1016/j.ejmech.2024.116828 Ravindar Lekkala 1 , Yan Hong Ng 2 , Shevin Rizal Feroz 2 , Nur Aqilah Zahirah Binti Norazmi 1 , Amatul Hamizah Ali 1 , Siti Aishah Hasbullah 1 , Norzila Ismail 3 , Hani Kartini Agustar 4 , Yee Ling Lau 5 , Nurul Izzaty Hassan 1
European Journal of Medicinal Chemistry ( IF 6.0 ) Pub Date : 2024-09-05 , DOI: 10.1016/j.ejmech.2024.116828 Ravindar Lekkala 1 , Yan Hong Ng 2 , Shevin Rizal Feroz 2 , Nur Aqilah Zahirah Binti Norazmi 1 , Amatul Hamizah Ali 1 , Siti Aishah Hasbullah 1 , Norzila Ismail 3 , Hani Kartini Agustar 4 , Yee Ling Lau 5 , Nurul Izzaty Hassan 1
Affiliation
|
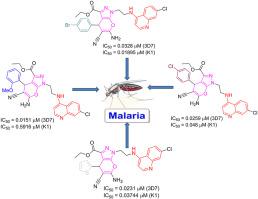
In this work, a series of nineteen novel pyrano[2,3-c]pyrazole-4-aminoquinoline hybrids were synthesized as potent antimalarial agents by covalently linking the scaffolds of 4-aminoquinoline and pyrano[2,3-c]pyrazoles via an ethyl linker and characterized using Fourier transform infrared spectroscopy (FTIR) and nuclear magnetic resonance spectroscopy (NMR). Molecular docking was used to test each hybrid's and standard chloroquine's ability to bind to Plasmodium falciparum lactate dehydrogenase enzyme (Pf LDH), an important enzyme in the parasite's glycolytic pathway. The hybrid compounds had a stronger binding affinity than the standard chloroquine (CQ). The schizontical antimalarial test of pyrano[2,3-c]pyrazole-4-aminoquinoline hybrid compound shows that all nineteen hybrid compounds were potent with the IC50 values ranging from 0.0151 to 0.301 μM against the CQ-sensitive 3D7 P. falciparum strain, and were active against the CQ-resistant K1 P. falciparum strain with the IC50 values ranging from 0.01895 to 2.746 μM. All the tested hybrid compounds were less potent than the standard drug chloroquine dipaspate (CQDP) against the CQ-sensitive 3D7 strain. In contrast, nine of the nineteen hybrids (16d , 16g , 16h , 16i , 16l , 16n , 16o , 16r, and 16s ) displayed superior antimalarial activity than the CQDP against the CQ-resistant K1 P. falciparum strain. Among all the tested hybrids, 16c against the 3D7 strain and 16h against the K1 strain were the most promising antimalarial agents with 0.0151 and 0.01895 μM of IC50 values, respectively. In addition, the compounds were selective, showing moderate to low cytotoxic activity against a human normal liver WRL68 cell line. The synthesis of pyrano[2,3-c]pyrazole-4-aminoquinoline hybrids introduces new chemical entities that have the potential to exhibit potent antimalarial activity. It could address the ongoing challenge of drug resistance in malaria treatment.
中文翻译:

吡喃并[2,3-c]吡唑-4-氨基喹啉杂交体作为有效抗疟化合物的设计与合成
在这项工作中,通过乙基接头共价连接 4-氨基喹啉和吡喃[2,3-c] 吡唑的支架,合成了一系列 19 种新型吡喃[2,3-c]吡唑-4-氨基喹啉杂交体作为有效的抗疟药,并使用傅里叶变换红外光谱 (FTIR) 和核磁共振波谱 (NMR) 进行表征。分子对接用于测试每个杂交体和标准氯喹与恶性疟原虫乳酸脱氢酶 (PfLDH) 结合的能力,PfLDH 是寄生虫糖酵解途径中的重要酶。杂交化合物比标准氯喹 (CQ) 具有更强的结合亲和力。吡喃[2,3-c]吡唑-4-氨基喹啉杂交化合物的裂殖抗疟试验显示,所有 19 种杂交化合物均有效,对 CQ 敏感的 3D7 恶性疟原虫菌株的 IC50 值为 0.0151 至 0.301 μM,对 CQ 耐药的 K1 恶性疟原虫菌株具有活性,IC50 值为 0.01895 至 2.746 μM。所有测试的杂交化合物对 CQ 敏感的 3D7 菌株的效力均低于标准药物二苯丙酸氯喹 (CQDP)。相比之下,19 个杂交种中有 9 个(16d、16g、16h、16i、16l、16n、16o、16r 和 16s)对 CQ 耐药的 K1 恶性疟原虫菌株表现出优于 CQDP 的抗疟活性。在所有测试的杂交种中,针对 3D7 菌株的 16c 和针对 K1 菌株的 16h 是最有前途的抗疟药,IC50 值分别为 0.0151 和 0.01895 μM。此外,这些化合物具有选择性,对人正常肝脏 WRL68 细胞系显示出中度至低度细胞毒活性。 吡喃[2,3-c]吡唑-4-氨基喹啉杂交体的合成引入了新的化学实体,这些实体有可能表现出有效的抗疟活性。它可以解决疟疾治疗中持续的耐药性挑战。
更新日期:2024-09-05
中文翻译:

吡喃并[2,3-c]吡唑-4-氨基喹啉杂交体作为有效抗疟化合物的设计与合成
在这项工作中,通过乙基接头共价连接 4-氨基喹啉和吡喃[2,3-c] 吡唑的支架,合成了一系列 19 种新型吡喃[2,3-c]吡唑-4-氨基喹啉杂交体作为有效的抗疟药,并使用傅里叶变换红外光谱 (FTIR) 和核磁共振波谱 (NMR) 进行表征。分子对接用于测试每个杂交体和标准氯喹与恶性疟原虫乳酸脱氢酶 (PfLDH) 结合的能力,PfLDH 是寄生虫糖酵解途径中的重要酶。杂交化合物比标准氯喹 (CQ) 具有更强的结合亲和力。吡喃[2,3-c]吡唑-4-氨基喹啉杂交化合物的裂殖抗疟试验显示,所有 19 种杂交化合物均有效,对 CQ 敏感的 3D7 恶性疟原虫菌株的 IC50 值为 0.0151 至 0.301 μM,对 CQ 耐药的 K1 恶性疟原虫菌株具有活性,IC50 值为 0.01895 至 2.746 μM。所有测试的杂交化合物对 CQ 敏感的 3D7 菌株的效力均低于标准药物二苯丙酸氯喹 (CQDP)。相比之下,19 个杂交种中有 9 个(16d、16g、16h、16i、16l、16n、16o、16r 和 16s)对 CQ 耐药的 K1 恶性疟原虫菌株表现出优于 CQDP 的抗疟活性。在所有测试的杂交种中,针对 3D7 菌株的 16c 和针对 K1 菌株的 16h 是最有前途的抗疟药,IC50 值分别为 0.0151 和 0.01895 μM。此外,这些化合物具有选择性,对人正常肝脏 WRL68 细胞系显示出中度至低度细胞毒活性。 吡喃[2,3-c]吡唑-4-氨基喹啉杂交体的合成引入了新的化学实体,这些实体有可能表现出有效的抗疟活性。它可以解决疟疾治疗中持续的耐药性挑战。































 京公网安备 11010802027423号
京公网安备 11010802027423号