当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Chemoselective Synthesis of α-Tertiary Hydroxy Oximes via Photochemical 1,3-Boronate Rearrangement
Organic Letters ( IF 4.9 ) Pub Date : 2024-09-09 , DOI: 10.1021/acs.orglett.4c02353 Ruijing Cai 1, 2 , Peng Zou 1 , Yixin Zhang 1 , Yiyun Chen 1, 2, 3
Organic Letters ( IF 4.9 ) Pub Date : 2024-09-09 , DOI: 10.1021/acs.orglett.4c02353 Ruijing Cai 1, 2 , Peng Zou 1 , Yixin Zhang 1 , Yiyun Chen 1, 2, 3
Affiliation
|
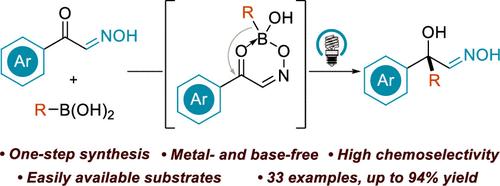
Tertiary alcohols with adjacent nucleophilic labile groups are prevalent in bioactive molecules but are challenging to synthesize. Herein we introduce a direct, protecting group-free method to access α-tertiary hydroxy oximes via photochemical 1,3-boronate rearrangement. This reaction delivers high yields (up to 94%) using readily available boronic acids, is scalable to gram quantities, and allows for further derivatization to other nitrogen-containing molecules.
中文翻译:

通过光化学 1,3-硼酸酯重排化学选择性合成 α-叔羟基肟
具有相邻亲核不稳定基团的叔醇在生物活性分子中普遍存在,但合成具有挑战性。在此,我们介绍了一种直接、无保护基团的方法,通过光化学 1,3-硼酸酯重排获得 α-叔羟基肟。该反应使用现成的硼酸实现高产率(高达 94%),可扩展到克级,并允许进一步衍生化为其他含氮分子。
更新日期:2024-09-09
中文翻译:

通过光化学 1,3-硼酸酯重排化学选择性合成 α-叔羟基肟
具有相邻亲核不稳定基团的叔醇在生物活性分子中普遍存在,但合成具有挑战性。在此,我们介绍了一种直接、无保护基团的方法,通过光化学 1,3-硼酸酯重排获得 α-叔羟基肟。该反应使用现成的硼酸实现高产率(高达 94%),可扩展到克级,并允许进一步衍生化为其他含氮分子。































 京公网安备 11010802027423号
京公网安备 11010802027423号