当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Bis(imidazolidine)-Derived NCN Nickel-Pincer-Catalyzed Asymmetric Reactions
Organic Letters ( IF 4.9 ) Pub Date : 2024-09-09 , DOI: 10.1021/acs.orglett.4c02773 Tomoya Yokota 1 , Yan Yu 1 , Kensuke Araseki 1 , Takayoshi Arai 1
Organic Letters ( IF 4.9 ) Pub Date : 2024-09-09 , DOI: 10.1021/acs.orglett.4c02773 Tomoya Yokota 1 , Yan Yu 1 , Kensuke Araseki 1 , Takayoshi Arai 1
Affiliation
|
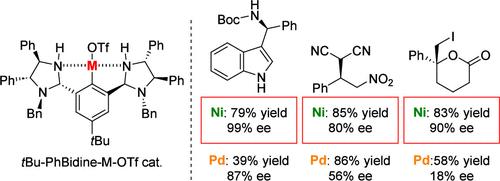
A bis(imidazolidine)-derived NCN nickel-pincer complex (tBu-PhBidine-Ni-OTf: NCN-Ni-OTf) was synthesized by the oxidative addition of imidazolidine-containing aryl triflate to Ni(cod)2 in MeCN. NCN-Ni-OTf exhibited asymmetric induction in three reactions. In the Friedel–Crafts reaction of indoles with N-Boc imines, 3-indolylmethanamine products were obtained in 79% yield with 99% ee. In a conjugate addition reaction of malononitrile to nitroalkenes, products were obtained in 95% yield with 75% ee. In iodolactonization, the pincer-Ni complex showed catalytic activity superior to that of tBu-PhBidine-Pd-OTf.
中文翻译:

双(咪唑烷)衍生的NCN镍钳催化的不对称反应
通过将含咪唑烷的芳基三氟甲磺酸酯氧化加成到 MeCN 中的 Ni(cod) 2上,合成了双(咪唑烷)衍生的 NCN 镍钳络合物 ( t Bu-PhBidine-Ni-OTf: NCN-Ni-OTf )。 NCN-Ni-OTf在三个反应中表现出不对称诱导。在吲哚与N -Boc 亚胺的弗里德尔-克来福特反应中,得到 3-吲哚基甲胺产物,收率 79%,ee 99%。在丙二腈与硝基烯烃的共轭加成反应中,产物收率为 95%,ee 为 75%。在碘内酯化反应中,钳-Ni配合物的催化活性优于t Bu-PhBidine-Pd-OTf。
更新日期:2024-09-09
中文翻译:

双(咪唑烷)衍生的NCN镍钳催化的不对称反应
通过将含咪唑烷的芳基三氟甲磺酸酯氧化加成到 MeCN 中的 Ni(cod) 2上,合成了双(咪唑烷)衍生的 NCN 镍钳络合物 ( t Bu-PhBidine-Ni-OTf: NCN-Ni-OTf )。 NCN-Ni-OTf在三个反应中表现出不对称诱导。在吲哚与N -Boc 亚胺的弗里德尔-克来福特反应中,得到 3-吲哚基甲胺产物,收率 79%,ee 99%。在丙二腈与硝基烯烃的共轭加成反应中,产物收率为 95%,ee 为 75%。在碘内酯化反应中,钳-Ni配合物的催化活性优于t Bu-PhBidine-Pd-OTf。































 京公网安备 11010802027423号
京公网安备 11010802027423号