当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Phosphine-Mediated Reductive Insertion of α-Keto Esters and Isatins into Phthalic Anhydride Derivatives
Organic Letters ( IF 4.9 ) Pub Date : 2024-09-09 , DOI: 10.1021/acs.orglett.4c02824 Yuefei Liu 1 , Qian Liu 1 , Rongfang Liu 2 , Xiaoqi Liu 1 , Hongyu Guo 1 , Wenjing Yang 1 , Rong Zhou 1, 3
Organic Letters ( IF 4.9 ) Pub Date : 2024-09-09 , DOI: 10.1021/acs.orglett.4c02824 Yuefei Liu 1 , Qian Liu 1 , Rongfang Liu 2 , Xiaoqi Liu 1 , Hongyu Guo 1 , Wenjing Yang 1 , Rong Zhou 1, 3
Affiliation
|
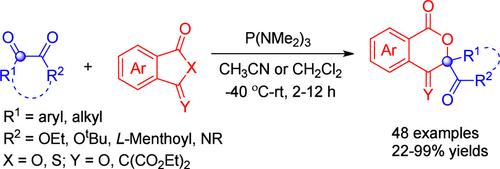
Herein, we report an unprecedented P(NMe2)3-mediated reductive insertion of 1,2-dicarbonyl compounds including α-keto esters and isatins into phthalic anhydride-derived alkenes and phthalic anhydrides, which furnishes the corresponding isochroman-1-ones and isochroman-1,4-diones, respectively, in moderate to excellent yields with high chemo- and regioselectivity. Furthermore, the asymmetric version of the ring expansion reaction could be realized by using a chiral auxiliary strategy. Mechanistically, the nucleophilic attack of the Kukhtin-Ramirez adduct, generated from P(NMe2)3 and 1,2-dicarbonyl compound, to the anhydride derivative, followed by a cascade ring-opening and ring-closure process, affords the ring expansion product. The reaction represents a novel metal-free carbon insertion ring expansion of aliphatic rings and also the first [1 + 5] annulation involving the Kukhtin-Ramirez adducts.
中文翻译:

膦介导的 α-酮酯和靛红还原插入邻苯二甲酸酐衍生物
在此,我们报道了前所未有的P(NMe 2 ) 3介导的1,2-二羰基化合物(包括α-酮酯和靛红)还原插入邻苯二甲酸酐衍生的烯烃和邻苯二甲酸酐中,提供了相应的异色满-1-酮和isochroman-1,4-diones 分别具有中等至优异的产率和高化学选择性和区域选择性。此外,扩环反应的不对称形式可以通过使用手性辅助策略来实现。从机理上讲,由 P(NMe 2 ) 3和 1,2-二羰基化合物生成的 Kukhtin-Ramirez 加合物对酸酐衍生物的亲核攻击,随后进行级联开环和闭环过程,从而实现环扩展产品。该反应代表了脂肪族环的新型无金属碳插入环扩展,也是涉及 Kukhtin-Ramirez 加合物的第一个 [1 + 5] 环化。
更新日期:2024-09-09
中文翻译:

膦介导的 α-酮酯和靛红还原插入邻苯二甲酸酐衍生物
在此,我们报道了前所未有的P(NMe 2 ) 3介导的1,2-二羰基化合物(包括α-酮酯和靛红)还原插入邻苯二甲酸酐衍生的烯烃和邻苯二甲酸酐中,提供了相应的异色满-1-酮和isochroman-1,4-diones 分别具有中等至优异的产率和高化学选择性和区域选择性。此外,扩环反应的不对称形式可以通过使用手性辅助策略来实现。从机理上讲,由 P(NMe 2 ) 3和 1,2-二羰基化合物生成的 Kukhtin-Ramirez 加合物对酸酐衍生物的亲核攻击,随后进行级联开环和闭环过程,从而实现环扩展产品。该反应代表了脂肪族环的新型无金属碳插入环扩展,也是涉及 Kukhtin-Ramirez 加合物的第一个 [1 + 5] 环化。































 京公网安备 11010802027423号
京公网安备 11010802027423号