当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Nickel-Catalyzed Secondary Benzylic C–O Bond Borylation
Organic Letters ( IF 4.9 ) Pub Date : 2024-09-09 , DOI: 10.1021/acs.orglett.4c02920 Pengfei Li 1 , Chenyan Zhang 1 , Jiaqi Wang 1 , Lei Zhang 1
Organic Letters ( IF 4.9 ) Pub Date : 2024-09-09 , DOI: 10.1021/acs.orglett.4c02920 Pengfei Li 1 , Chenyan Zhang 1 , Jiaqi Wang 1 , Lei Zhang 1
Affiliation
|
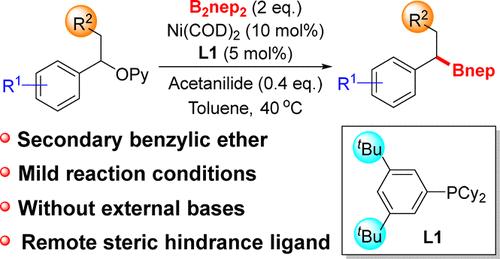
A remote steric hindrance ligand (m-tBu)2C6H3PCy2 (L1) was synthesized to promote Ni-catalyzed C–O bond activation. The reaction achieved high yields for secondary benzylic C(sp3)–O borylation in non-π-extended systems under mild conditions. Mechanistic studies indicate that the nickel complex containing 1 equiv of L1 serves as the active catalyst, while increased loading of L1 gives the inactive bisligated Ni species. Acetanilide is crucial for the cross-coupling reaction, which facilitates generation of the monoligated nickel species.
中文翻译:

镍催化的二次苄基 C-O 键硼化反应
合成了远程空间位阻配体( m - t Bu) 2 C 6 H 3 PCy 2 ( L1 )来促进Ni催化的C-O键活化。该反应在温和条件下在非π-延伸体系中实现了仲苄基C(sp 3 )–O硼基化的高产率。机理研究表明,含有 1 当量L1的镍络合物充当活性催化剂,而增加L1的负载量则产生非活性双配位镍物种。乙酰苯胺对于交叉偶联反应至关重要,它有助于单配位镍物质的生成。
更新日期:2024-09-09
中文翻译:

镍催化的二次苄基 C-O 键硼化反应
合成了远程空间位阻配体( m - t Bu) 2 C 6 H 3 PCy 2 ( L1 )来促进Ni催化的C-O键活化。该反应在温和条件下在非π-延伸体系中实现了仲苄基C(sp 3 )–O硼基化的高产率。机理研究表明,含有 1 当量L1的镍络合物充当活性催化剂,而增加L1的负载量则产生非活性双配位镍物种。乙酰苯胺对于交叉偶联反应至关重要,它有助于单配位镍物质的生成。































 京公网安备 11010802027423号
京公网安备 11010802027423号