当前位置:
X-MOL 学术
›
Sens. Actuators B Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Dual-emissive near-infrared fluorescent probe revealing fluidity changes in lysosomal membranes during lysosomal fission and fusion
Sensors and Actuators B: Chemical ( IF 8.0 ) Pub Date : 2024-08-30 , DOI: 10.1016/j.snb.2024.136550 Huina Wang , Chenyu Shi , Baoli Dong , Minggang Tian
Sensors and Actuators B: Chemical ( IF 8.0 ) Pub Date : 2024-08-30 , DOI: 10.1016/j.snb.2024.136550 Huina Wang , Chenyu Shi , Baoli Dong , Minggang Tian
|
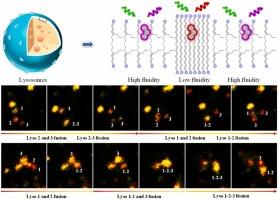
Lysosomal membranes fluidity is a crucial parameter closely relative to lysosomal fission and fusion, autophagy, endocytosis, and exocytosis. However, fluorescent probes for the visualization of lysosomal membranes fluidity were rarely reported. In this work, by linking an amine moiety on a polarity-responsive near-infrared fluorophore, we have constructed a well membrane permeable probe (DMA) to visualize the changes in lysosomal membranes fluidity in dual-channel mode. GUVs and lipid treatment experiments demonstrated that DMA enabled visualization of the increase and decrease of lysosomal membrane fluidity with red-shifted and blue-shifted emission, respectively. With the unique probe, the up-regulated fluidity of lysosomal membranes under starvation in buffer was revealed for the first time. The increased fluidity should be attributed to the lysosomal membrane fusing with other organelles facilitated by the treatment at low level of amino acids. The firstly increased and then decreased lysosomal membranes fluidity during ferroptosis were successfully observed. Particularly, the changes in membrane fluidity were in-situ and real-timely visualized during lysosomal fission and fusion. Lysosomal membrane fluidity was initially up-regulated before lysosomal fusion, and down-regulated after the fusion procedure was completed. The membrane fluidity was also up-regulated after lysosomal fission.
中文翻译:

双发射近红外荧光探针,揭示溶酶体裂变和融合过程中溶酶体膜的流动性变化
溶酶体膜流动性是与溶酶体裂变和融合、自噬、内吞作用和胞吐作用密切相关的关键参数。然而,用于溶酶体膜流动性可视化的荧光探针很少见报道。在这项工作中,通过将胺部分连接到极性响应的近红外荧光团上,我们构建了一个孔膜渗透探针 (DMA),以可视化双通道模式下溶酶体膜流动性的变化。GUV 和脂质处理实验表明,DMA 能够分别通过红移和蓝移发射可视化溶酶体膜流动性的增加和减少。使用独特的探针,首次揭示了溶酶体膜在缓冲液中饥饿下上调的流动性。流动性的增加应归因于溶酶体膜与其他细胞器融合,这在低水平氨基酸的处理下得到了促进。成功观察到铁死亡过程中溶酶体膜流动性首先增加,然后降低。特别是,在溶酶体裂变和融合过程中,膜流动性的变化是原位和实时可视化的。溶酶体膜流动性最初在溶酶体融合前上调,在融合过程完成后下调。溶酶体裂变后膜流动性也上调。
更新日期:2024-08-30
中文翻译:

双发射近红外荧光探针,揭示溶酶体裂变和融合过程中溶酶体膜的流动性变化
溶酶体膜流动性是与溶酶体裂变和融合、自噬、内吞作用和胞吐作用密切相关的关键参数。然而,用于溶酶体膜流动性可视化的荧光探针很少见报道。在这项工作中,通过将胺部分连接到极性响应的近红外荧光团上,我们构建了一个孔膜渗透探针 (DMA),以可视化双通道模式下溶酶体膜流动性的变化。GUV 和脂质处理实验表明,DMA 能够分别通过红移和蓝移发射可视化溶酶体膜流动性的增加和减少。使用独特的探针,首次揭示了溶酶体膜在缓冲液中饥饿下上调的流动性。流动性的增加应归因于溶酶体膜与其他细胞器融合,这在低水平氨基酸的处理下得到了促进。成功观察到铁死亡过程中溶酶体膜流动性首先增加,然后降低。特别是,在溶酶体裂变和融合过程中,膜流动性的变化是原位和实时可视化的。溶酶体膜流动性最初在溶酶体融合前上调,在融合过程完成后下调。溶酶体裂变后膜流动性也上调。































 京公网安备 11010802027423号
京公网安备 11010802027423号