Diabetologia ( IF 8.4 ) Pub Date : 2024-09-09 , DOI: 10.1007/s00125-024-06161-0 Nathalie Esser 1, 2, 3, 4 , Meghan F Hogan 1, 2 , Andrew T Templin 1, 2, 5 , Rehana Akter 1, 2 , Brendy S Fountaine 1 , Joseph J Castillo 1, 2 , Assam El-Osta 6 , Lakshan Manathunga 7, 8 , Alexander Zhyvoloup 9 , Daniel P Raleigh 7, 8, 9 , Sakeneh Zraika 1, 2 , Rebecca L Hull 1, 2 , Steven E Kahn 1, 2
|
Aims/hypothesis
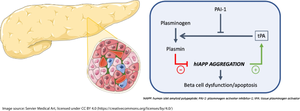
Apart from its fibrinolytic activity, the tissue plasminogen activator (tPA)/plasmin system has been reported to cleave the peptide amyloid beta, attenuating brain amyloid deposition in Alzheimer’s disease. As aggregation of human islet amyloid polypeptide (hIAPP) is toxic to beta cells, we sought to determine whether activation of the fibrinolytic system can also reduce islet amyloid deposition and its cytotoxic effects, which are both observed in type 2 diabetes.
Methods
The expression of Plat (encoding tPA) and plasmin activity were measured in isolated islets from amyloid-prone hIAPP transgenic mice or non-transgenic control islets expressing non-amyloidogenic mouse islet amyloid polypeptide cultured in the absence or presence of the amyloid inhibitor Congo Red. Plat expression was also determined in hIAPP-treated primary islet endothelial cells, bone marrow-derived macrophages (BMDM) and INS-1 cells, in order to determine the islet cell type(s) producing tPA in response to hIAPP aggregation. Cell-free thioflavin-T assays and MS were used to respectively monitor hIAPP aggregation kinetics and investigate plasmin cleavage of hIAPP. Cell viability was assessed in INS-1 beta cells treated with hIAPP with or without plasmin. Finally, to confirm the findings in human samples, PLAT expression was measured in freshly isolated islets from donors with and without type 2 diabetes.
Results
In isolated islets from transgenic mice, islet Plat expression and plasmin activity increased significantly with the process of amyloid deposition (p≤0.01, n=5); these effects were not observed in islets from non-transgenic mice and were blocked by Congo Red (p≤0.01, n=4). In response to hIAPP exposure, Plat expression increased in BMDM and INS-1 cells vs vehicle-treated cells (p≤0.05, n=4), but not in islet endothelial cells. Plasmin reduced hIAPP fibril formation in a dose-dependent manner in a cell-free system, and restored hIAPP-induced loss of cell viability in INS-1 beta cells (p≤0.01, n=5). Plasmin cleaved monomeric hIAPP, inducing a rapid decrease in the abundance of full-length hIAPP and the appearance of hIAPP 1–11 and 12–37 fragments. hIAPP 12–37, which contains the critical amyloidogenic region, was not toxic to INS-1 cells. Finally, PLAT expression was significantly increased by 2.4-fold in islets from donors with type 2 diabetes (n=4) vs islets from donors without type 2 diabetes (n=7) (p≤0.05).
Conclusions/interpretation
The fibrinolytic system is upregulated in islets with hIAPP aggregation. Plasmin rapidly degrades hIAPP, limiting its aggregation into amyloid and thus protecting beta cells from hIAPP-induced toxicity. Thus, increasing islet plasmin activity might be a strategy to limit beta cell loss in type 2 diabetes.
Graphical Abstract
中文翻译:

胰岛组织纤溶酶原激活剂/纤溶酶系统因人胰岛淀粉样蛋白多肽聚集而上调,并保护 β 细胞免受聚集诱导的毒性
目标/假设
据报道,除了纤溶活性外,组织纤溶酶原激活剂 (tPA)/纤溶酶系统还可以裂解肽淀粉样蛋白 β,从而减弱阿尔茨海默病中的脑淀粉样蛋白沉积。由于人胰岛淀粉样蛋白多肽 (hIAPP) 的聚集对 β 细胞有毒,我们试图确定纤溶系统的激活是否也能减少胰岛淀粉样蛋白沉积及其细胞毒作用,这两者都在 2 型糖尿病中观察到。
方法
在不存在或存在淀粉样蛋白抑制剂刚果红的情况下,在淀粉样蛋白易感 hIAPP 转基因小鼠或表达非淀粉样蛋白小鼠胰岛淀粉样蛋白多肽的非转基因对照胰岛的分离胰岛中测量 Plat(编码 tPA)和纤溶酶活性的表达。还测定了 hIAPP 处理的原代胰岛内皮细胞、骨髓衍生的巨噬细胞 (BMDM) 和 INS-1 细胞中的板表达,以确定响应 hIAPP 聚集而产生 tPA 的胰岛细胞类型。游离硫黄素-T 检测和 MS 分别监测 hIAPP 聚集动力学并研究 hIAPP 的纤溶酶裂解。在用含或不含纤溶酶的 hIAPP 处理的 INS-1 β 细胞中评估细胞活力。最后,为了证实人类样本中的发现,在患有和不患有 2 型糖尿病的供体的新鲜分离的胰岛中测量了 PLAT 表达。
结果
在转基因小鼠的分离胰岛中,胰岛 Plat 表达和纤溶酶活性随着淀粉样蛋白沉积过程而显著增加 (p≤0.01, n=5);这些效应在非转基因小鼠的胰岛中未观察到,并且被刚果红阻断 (p≤0.01, n=4)。作为对 hIAPP 暴露的响应,BMDM 和 INS-1 细胞中的铂表达与载体处理的细胞 (p≤0.05, n=4) 相比增加,但在胰岛内皮细胞中没有增加。纤溶酶在无细胞系统中以剂量依赖性方式减少 hIAPP 原纤维的形成,并恢复 hIAPP 诱导的 INS-1 β 细胞活力损失 (p≤0.01, n=5)。纤溶酶裂解单体 hIAPP,诱导全长 hIAPP 丰度迅速降低,并出现 hIAPP 1-11 和 12-37 片段。hIAPP 12-37 包含关键的淀粉样蛋白生成区域,对 INS-1 细胞无毒。最后,与非 2 型糖尿病供体 (n=7) 相比,2 型糖尿病供体胰岛 (n=4) 的 PLAT 表达显著增加 2.4 倍 (p≤0.05)。
结论/解释
纤溶系统在具有 hIAPP 聚集的胰岛中上调。纤溶酶可快速降解 hIAPP,限制其聚集成淀粉样蛋白,从而保护 β 细胞免受 hIAPP 诱导的毒性。因此,增加胰岛纤溶酶活性可能是限制 2 型糖尿病中 β 细胞丢失的一种策略。

































 京公网安备 11010802027423号
京公网安备 11010802027423号