当前位置:
X-MOL 学术
›
J. Mater. Chem. A
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Efficient solar-driven electrocatalytic nitrate-to-ammonia conversion by 2D ultrathin Fe single-atom catalysts
Journal of Materials Chemistry A ( IF 10.7 ) Pub Date : 2024-09-09 , DOI: 10.1039/d4ta03824j Ji Li 1, 2 , Weiqi Zhong 1 , Kai Wu 1 , Eddy Petit 2 , Luc Lajaunie 3, 4 , Kun Qi 5 , Yang Zhang 2 , Huali Wu 6 , Jiefeng Liu 2 , Jing Heng 1 , Xuechuan Wang 1 , Qingxin Han 1 , Taotao Qiang 1 , Damien Voiry 2
Journal of Materials Chemistry A ( IF 10.7 ) Pub Date : 2024-09-09 , DOI: 10.1039/d4ta03824j Ji Li 1, 2 , Weiqi Zhong 1 , Kai Wu 1 , Eddy Petit 2 , Luc Lajaunie 3, 4 , Kun Qi 5 , Yang Zhang 2 , Huali Wu 6 , Jiefeng Liu 2 , Jing Heng 1 , Xuechuan Wang 1 , Qingxin Han 1 , Taotao Qiang 1 , Damien Voiry 2
Affiliation
|
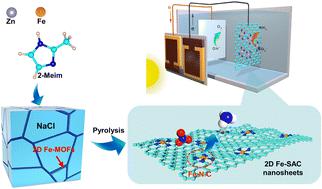
The controllable design of single-atom electrocatalysts with high active site exposure density, enhanced mass/volume specific activity, and low mass transfer resistance holds tremendous potential for green ammonia synthesis involving the electrochemical nitrate reduction reaction (eNO3RR). Here we report the synthesis of ultrathin two-dimensional electrocatalysts with the inclusion of iron (Fe) single-atom catalytic active sites (2D Fe-SACs) for the nitrate reduction reaction (NO3RR). Our isotopic nuclear magnetic resonance (NMR) analyses revealed that 2D Fe-SACs exhibit remarkable performance, with a maximum faradaic efficiency of 95.4 ± 4.00% for the NO3RR to NH3 at an overpotential of −0.40 V versus the reversible hydrogen electrode (vs. RHE). Density functional theory (DFT) calculations suggest that the enhanced selectivity of 2D Fe-SACs to produce NH3 is attributed to a low energy barrier of 0.31 eV associated with the oxidation of *NO to *NHO. Then, we assembled the catalyst in a two-electrode electrolyzer connected to an InGaP/GaAs/Ge triple-junction solar cell and achieved a solar-to-ammonia (STA) conversion efficiency of 4.35% and a maximum yield rate of 0.29 mmol h−1 cm−2 equivalent to 5.10 mg h−1 cm−2. These findings open new avenues for developing platinum group metal (PGM)-free single-atom catalysts (SACs) to realize the Haber-Bosch process using solar energy.
中文翻译:

通过二维超薄铁单原子催化剂实现高效太阳能驱动的电催化硝酸盐至氨的转化
单原子电催化剂的可控设计具有高活性位点暴露密度、增强的质量/体积比活性和低传质阻力,为涉及电化学硝酸盐还原反应(eNO 3 RR)的绿色氨合成提供了巨大的潜力。在这里,我们报道了用于硝酸盐还原反应(NO 3 RR)的超薄二维电催化剂的合成,其中包含铁(Fe)单原子催化活性位点(2D Fe-SAC)。我们的同位素核磁共振 (NMR) 分析表明,2D Fe-SAC 表现出卓越的性能,相对于可逆氢电极,在 -0.40 V 的过电势下,NO 3 RR 到 NH 3的最大法拉第效率为 95.4 ± 4.00%(与RHE)。密度泛函理论(DFT) 计算表明,2D Fe-SAC 产生NH 3的选择性增强归因于与*NO 氧化为*NHO 相关的0.31 eV 低能垒。然后,我们将催化剂组装在连接到InGaP/GaAs/Ge三结太阳能电池的两电极电解槽中,实现了4.35%的太阳能-氨(STA)转换效率和0.29 mmol h的最大产率-1 cm -2相当于5.10 mg h -1 cm -2 。这些发现为开发不含铂族金属(PGM)的单原子催化剂(SAC)以实现利用太阳能的哈伯-博世工艺开辟了新途径。
更新日期:2024-09-09
中文翻译:

通过二维超薄铁单原子催化剂实现高效太阳能驱动的电催化硝酸盐至氨的转化
单原子电催化剂的可控设计具有高活性位点暴露密度、增强的质量/体积比活性和低传质阻力,为涉及电化学硝酸盐还原反应(eNO 3 RR)的绿色氨合成提供了巨大的潜力。在这里,我们报道了用于硝酸盐还原反应(NO 3 RR)的超薄二维电催化剂的合成,其中包含铁(Fe)单原子催化活性位点(2D Fe-SAC)。我们的同位素核磁共振 (NMR) 分析表明,2D Fe-SAC 表现出卓越的性能,相对于可逆氢电极,在 -0.40 V 的过电势下,NO 3 RR 到 NH 3的最大法拉第效率为 95.4 ± 4.00%(与RHE)。密度泛函理论(DFT) 计算表明,2D Fe-SAC 产生NH 3的选择性增强归因于与*NO 氧化为*NHO 相关的0.31 eV 低能垒。然后,我们将催化剂组装在连接到InGaP/GaAs/Ge三结太阳能电池的两电极电解槽中,实现了4.35%的太阳能-氨(STA)转换效率和0.29 mmol h的最大产率-1 cm -2相当于5.10 mg h -1 cm -2 。这些发现为开发不含铂族金属(PGM)的单原子催化剂(SAC)以实现利用太阳能的哈伯-博世工艺开辟了新途径。

































 京公网安备 11010802027423号
京公网安备 11010802027423号