当前位置:
X-MOL 学术
›
J. Mater. Chem. A
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Boosting Lewis acidic sites via SMSI to enrich oxygen vacancies on Pt–CeO2 catalysts: enhancing efficiency of CO promoted toluene catalysis
Journal of Materials Chemistry A ( IF 10.7 ) Pub Date : 2024-09-09 , DOI: 10.1039/d4ta04344h Xiaoying Zhou 1 , Mingyuan Zhang 1 , Peiqi Mao 1 , Jiaxuan Pang 1 , Jiayi Wang 2 , Liping Wang 1 , Chentao Hou 1 , Daiqi Ye 3
Journal of Materials Chemistry A ( IF 10.7 ) Pub Date : 2024-09-09 , DOI: 10.1039/d4ta04344h Xiaoying Zhou 1 , Mingyuan Zhang 1 , Peiqi Mao 1 , Jiaxuan Pang 1 , Jiayi Wang 2 , Liping Wang 1 , Chentao Hou 1 , Daiqi Ye 3
Affiliation
|
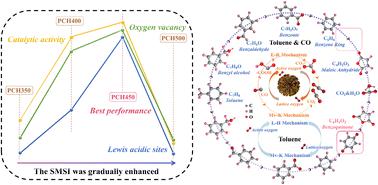
The optimization of electron transfer on Pt–CeO2 (PCH) was achieved by adjusting strong metal–support interaction (SMSI). With an increase in SMSI, there was an initial increase followed by a decline in the concentration of total acid sites and oxygen vacancies (Ov) on PCH. The activity of PCH showed the same trend. Lewis acid is the main acid site of PCH, which facilitates the activation of lattice oxygen and the formation of Pt0 species, which improves the oxygen cycling ability of PCH. Brønsted acid assists in the formation of Ov. PCH450 showed the best co-catalytic activity for toluene and CO due to its highest Brønsted/Lewis acid value (0.211) and abundant Ov. At a 90% conversion rate (T90), the conversion temperature of toluene in mixed pollutants is advanced by 15 °C and that of CO is delayed by 28 °C. The findings of in situ DRIFTS indicated that CO shortens the reaction path of toluene, and toluene reduces the reaction rate of CO. Therefore, CO promotes toluene catalysis on PCH. Despite the competitive adsorption of mixed pollutants in the co-catalytic system, their reaction pathways remain independent. This study provides a theoretical basis for the thermal catalytic technology of industrial waste gas.
中文翻译:

通过 SMSI 增强路易斯酸性位点以富集 Pt-CeO2 催化剂上的氧空位:提高 CO 促进甲苯催化的效率
通过调整强金属-载体相互作用(SMSI)实现了 Pt-CeO 2 (PCH) 上电子转移的优化。随着SMSI的增加,PCH上总酸位点和氧空位(O v )的浓度先增加后减少。 PCH的活性也呈现出同样的趋势。 Lewis酸是PCH的主要酸位,有利于晶格氧的活化和Pt 0物种的形成,从而提高了PCH的氧循环能力。布朗斯台德酸有助于 O v的形成。 PCH450 由于具有最高的 Brønsted/Lewis 酸值 (0.211) 和丰富的 O v ,对甲苯和 CO 显示出最佳的助催化活性。在90%转化率( T 90 )下,混合污染物中甲苯的转化温度提前15℃,CO的转化温度延迟28℃。原位DRIFTS结果表明,CO缩短了甲苯的反应路径,而甲苯降低了CO的反应速率。因此,CO促进了甲苯对PCH的催化。尽管共催化系统中混合污染物存在竞争吸附,但它们的反应途径仍然独立。该研究为工业废气热催化技术提供了理论基础。
更新日期:2024-09-09
中文翻译:

通过 SMSI 增强路易斯酸性位点以富集 Pt-CeO2 催化剂上的氧空位:提高 CO 促进甲苯催化的效率
通过调整强金属-载体相互作用(SMSI)实现了 Pt-CeO 2 (PCH) 上电子转移的优化。随着SMSI的增加,PCH上总酸位点和氧空位(O v )的浓度先增加后减少。 PCH的活性也呈现出同样的趋势。 Lewis酸是PCH的主要酸位,有利于晶格氧的活化和Pt 0物种的形成,从而提高了PCH的氧循环能力。布朗斯台德酸有助于 O v的形成。 PCH450 由于具有最高的 Brønsted/Lewis 酸值 (0.211) 和丰富的 O v ,对甲苯和 CO 显示出最佳的助催化活性。在90%转化率( T 90 )下,混合污染物中甲苯的转化温度提前15℃,CO的转化温度延迟28℃。原位DRIFTS结果表明,CO缩短了甲苯的反应路径,而甲苯降低了CO的反应速率。因此,CO促进了甲苯对PCH的催化。尽管共催化系统中混合污染物存在竞争吸附,但它们的反应途径仍然独立。该研究为工业废气热催化技术提供了理论基础。































 京公网安备 11010802027423号
京公网安备 11010802027423号