当前位置:
X-MOL 学术
›
Energy Environ. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Identifying iodide-ion regulation of early-stage zinc nucleation and growth for high-rate anode-free zinc metal batteries
Energy & Environmental Science ( IF 32.4 ) Pub Date : 2024-09-09 , DOI: 10.1039/d4ee02784a Wenchao Shi 1 , Zhenjun Song 2 , Wenwei Zhang 1 , Sitian Lian 1 , Fuzhi Huang 1 , Qinyou An 1 , Qi Li 3
Energy & Environmental Science ( IF 32.4 ) Pub Date : 2024-09-09 , DOI: 10.1039/d4ee02784a Wenchao Shi 1 , Zhenjun Song 2 , Wenwei Zhang 1 , Sitian Lian 1 , Fuzhi Huang 1 , Qinyou An 1 , Qi Li 3
Affiliation
|
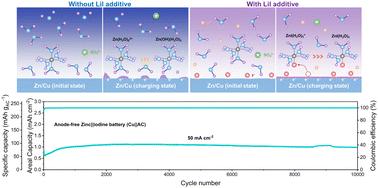
Anode-free aqueous zinc (Zn) metal batteries (AZMBs) have the advantage of providing higher energy density. However, without excess Zn metal, their cycling life is highly dependent on the reversibility of Zn deposition/dissolution, which is influenced by interfacial issues such as Zn dendrite formation and parasitic side reactions. A simple approach is developed to tackle this challenge by introducing lithium iodide as an additive to the electrolyte, where iodide ions (I−) play a crucial role in regulating the early-stage Zn nucleation and growth. Initially, the formation of an I−-rich electrochemical double layer reduces the Marcus charge transfer energy barrier of Zn ions (Zn2+), hence significantly lowering the heterogeneous nucleation overpotential of Zn. Subsequently, I− ions are preferentially adsorbed onto the Zn (100) and Zn (101) crystal planes compared to the Zn (002) plane, thereby promoting the Zn growth onto these two planes and leading to Zn plating with the dominating Zn (002) orientation. As a result, highly reversible Zn deposition/dissolution is achieved in the Zn‖copper battery, with a superior initial and average coulombic efficiency of 99.9%. Moreover, the anode-free Zn‖iodine battery demonstrates excellent cycling stability and ultra-high-rate performance (0.99 mA h cm−2 capacity retained corresponding to an 88.2% retention after 10 000 cycles at 50 mA cm−2). The I− regulation strategy for early-stage Zn nucleation and growth behavior provides a simple and innovative approach to improving the Zn deposition/dissolution interfacial stability and hence the cycling stability and rate capability of anode-free AZMBs and can also be extended to other anode-free metal batteries.
中文翻译:

确定高倍率无阳极锌金属电池早期锌成核和生长的碘离子调节
无阳极水性锌(Zn)金属电池(AZMB)具有提供更高能量密度的优点。然而,如果没有过量的锌金属,它们的循环寿命高度依赖于锌沉积/溶解的可逆性,而锌沉积/溶解的可逆性受到锌枝晶形成和寄生副反应等界面问题的影响。通过在电解质中引入碘化锂作为添加剂,开发了一种简单的方法来应对这一挑战,其中碘离子 (I - ) 在调节早期 Zn 成核和生长方面发挥着至关重要的作用。最初,富I -电化学双层的形成降低了Zn离子(Zn 2+ )的马库斯电荷转移能垒,从而显着降低了Zn的异相成核过电势。随后,与 Zn (002) 晶面相比,I -离子优先吸附到 Zn (100) 和 Zn (101) 晶面上,从而促进 Zn 在这两个晶面上生长,并导致 Zn (002) 占主导地位的 Zn 镀层) 方向。因此,Zn‖铜电池实现了高度可逆的锌沉积/溶解,初始库仑效率和平均库仑效率高达 99.9%。此外,无阳极锌碘电池表现出优异的循环稳定性和超高倍率性能(在50 mA cm -2下循环10 000次后容量保留为0.99 mA h cm -2 ,相当于88.2%的保留率)。 针对早期 Zn 成核和生长行为的I−调节策略提供了一种简单且创新的方法来提高 Zn 沉积/溶解界面稳定性,从而提高无阳极 AZMB 的循环稳定性和倍率性能,并且也可以扩展到其他阳极- 不含金属电池。
更新日期:2024-09-09
中文翻译:

确定高倍率无阳极锌金属电池早期锌成核和生长的碘离子调节
无阳极水性锌(Zn)金属电池(AZMB)具有提供更高能量密度的优点。然而,如果没有过量的锌金属,它们的循环寿命高度依赖于锌沉积/溶解的可逆性,而锌沉积/溶解的可逆性受到锌枝晶形成和寄生副反应等界面问题的影响。通过在电解质中引入碘化锂作为添加剂,开发了一种简单的方法来应对这一挑战,其中碘离子 (I - ) 在调节早期 Zn 成核和生长方面发挥着至关重要的作用。最初,富I -电化学双层的形成降低了Zn离子(Zn 2+ )的马库斯电荷转移能垒,从而显着降低了Zn的异相成核过电势。随后,与 Zn (002) 晶面相比,I -离子优先吸附到 Zn (100) 和 Zn (101) 晶面上,从而促进 Zn 在这两个晶面上生长,并导致 Zn (002) 占主导地位的 Zn 镀层) 方向。因此,Zn‖铜电池实现了高度可逆的锌沉积/溶解,初始库仑效率和平均库仑效率高达 99.9%。此外,无阳极锌碘电池表现出优异的循环稳定性和超高倍率性能(在50 mA cm -2下循环10 000次后容量保留为0.99 mA h cm -2 ,相当于88.2%的保留率)。 针对早期 Zn 成核和生长行为的I−调节策略提供了一种简单且创新的方法来提高 Zn 沉积/溶解界面稳定性,从而提高无阳极 AZMB 的循环稳定性和倍率性能,并且也可以扩展到其他阳极- 不含金属电池。































 京公网安备 11010802027423号
京公网安备 11010802027423号