当前位置:
X-MOL 学术
›
Chem. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Four-step continuous-flow total synthesis of (−)-debromoflustramine B using a chiral heterogeneous Pd NP catalyst
Chemical Science ( IF 7.6 ) Pub Date : 2024-09-09 , DOI: 10.1039/d4sc03471f Junwen Wang 1 , Feng Liang 1 , Zhen Dong 1 , Junrong Huang 1 , Yuxiang Zhu 2 , Hengzhi You 1, 3 , Fen-Er Chen 1, 3, 4
Chemical Science ( IF 7.6 ) Pub Date : 2024-09-09 , DOI: 10.1039/d4sc03471f Junwen Wang 1 , Feng Liang 1 , Zhen Dong 1 , Junrong Huang 1 , Yuxiang Zhu 2 , Hengzhi You 1, 3 , Fen-Er Chen 1, 3, 4
Affiliation
|
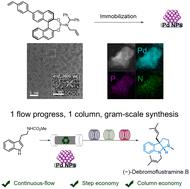
Various prenylated indoline alkaloids with diverse biological activities, including (−)-debromoflustramine B with significant butyrylcholinesterase inhibitory activity, could be synthesized by dearomative prenylation reactions of tryptophan derivatives. However, previously reported dearomative prenylations were limited to batch reactions at the milligram scale, requiring multistep reactions and complex post-processing to obtain the desired natural products. The more efficient synthesis of alkaloids remains challenging, as does the recovery of expensive catalysts. Herein, we developed a chiral heterogeneous Pd nanoparticle (NP) catalyst supported on a polymer, which produces indoline alkaloids in high yields with excellent enantioselectivities. Additionally, the first gram-scale four-step continuous-flow total synthesis of (−)-debromoflustramine B was successfully achieved with this chiral Pd heterogeneous catalyst, requiring only a simple post-processing step.
中文翻译:

使用手性非均相 Pd NP 催化剂四步连续流全合成 (−)-去溴氟曲明 B
通过色氨酸衍生物的脱芳香异戊二烯化反应可以合成具有多种生物活性的各种异戊二烯化二氢吲哚生物碱,包括具有显着丁酰胆碱酯酶抑制活性的(−)-去溴氟曲明B。然而,之前报道的脱芳异戊二烯化仅限于毫克级的批量反应,需要多步反应和复杂的后处理才能获得所需的天然产物。更有效地合成生物碱仍然具有挑战性,昂贵的催化剂的回收也是如此。在此,我们开发了一种负载在聚合物上的手性非均相钯纳米粒子(NP)催化剂,该催化剂能够以高产率生产具有优异对映选择性的二氢吲哚生物碱。此外,利用这种手性钯多相催化剂成功实现了(−)-去溴氟曲明B的首次克级四步连续流全合成,仅需要简单的后处理步骤。
更新日期:2024-09-09
中文翻译:

使用手性非均相 Pd NP 催化剂四步连续流全合成 (−)-去溴氟曲明 B
通过色氨酸衍生物的脱芳香异戊二烯化反应可以合成具有多种生物活性的各种异戊二烯化二氢吲哚生物碱,包括具有显着丁酰胆碱酯酶抑制活性的(−)-去溴氟曲明B。然而,之前报道的脱芳异戊二烯化仅限于毫克级的批量反应,需要多步反应和复杂的后处理才能获得所需的天然产物。更有效地合成生物碱仍然具有挑战性,昂贵的催化剂的回收也是如此。在此,我们开发了一种负载在聚合物上的手性非均相钯纳米粒子(NP)催化剂,该催化剂能够以高产率生产具有优异对映选择性的二氢吲哚生物碱。此外,利用这种手性钯多相催化剂成功实现了(−)-去溴氟曲明B的首次克级四步连续流全合成,仅需要简单的后处理步骤。































 京公网安备 11010802027423号
京公网安备 11010802027423号