Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Targeting APT2 improves MAVS palmitoylation and antiviral innate immunity
Molecular Cell ( IF 14.5 ) Pub Date : 2024-09-09 , DOI: 10.1016/j.molcel.2024.08.014 Lang Bu 1 , Huan Wang 2 , Shuishen Zhang 3 , Yi Zhang 2 , Miaowen Liu 2 , Zhengkun Zhang 2 , Xueji Wu 2 , Qiwei Jiang 2 , Lei Wang 2 , Wei Xie 2 , Miao He 4 , Zhengran Zhou 5 , Chao Cheng 3 , Jianping Guo 2
Molecular Cell ( IF 14.5 ) Pub Date : 2024-09-09 , DOI: 10.1016/j.molcel.2024.08.014 Lang Bu 1 , Huan Wang 2 , Shuishen Zhang 3 , Yi Zhang 2 , Miaowen Liu 2 , Zhengkun Zhang 2 , Xueji Wu 2 , Qiwei Jiang 2 , Lei Wang 2 , Wei Xie 2 , Miao He 4 , Zhengran Zhou 5 , Chao Cheng 3 , Jianping Guo 2
Affiliation
|
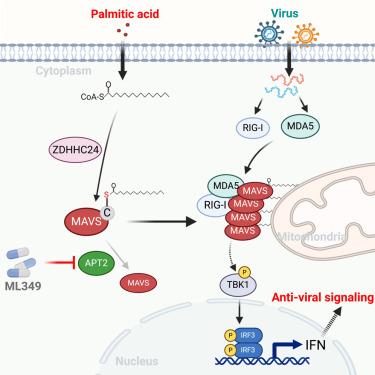
Innate immunity serves as the primary defense against viral and microbial infections in humans. The precise influence of cellular metabolites, especially fatty acids, on antiviral innate immunity remains largely elusive. Here, through screening a metabolite library, palmitic acid (PA) has been identified as a key modulator of antiviral infections in human cells. Mechanistically, PA induces mitochondrial antiviral signaling protein (MAVS) palmitoylation, aggregation, and subsequent activation, thereby enhancing the innate immune response. The palmitoyl-transferase ZDHHC24 catalyzes MAVS palmitoylation, thereby boosting the TBK1-IRF3-interferon (IFN) pathway, particularly under conditions of PA stimulation or high-fat-diet-fed mouse models, leading to antiviral immune responses. Additionally, APT2 de-palmitoylates MAVS, thus inhibiting antiviral signaling, suggesting that its inhibitors, such as ML349, effectively reverse MAVS activation in response to antiviral infections. These findings underscore the critical role of PA in regulating antiviral innate immunity through MAVS palmitoylation and provide strategies for enhancing PA intake or targeting APT2 for combating viral infections.
中文翻译:

靶向 APT2 可改善 MAVS 棕榈酰化和抗病毒先天免疫
先天免疫是人类抵御病毒和微生物感染的主要防御措施。细胞代谢物,尤其是脂肪酸,对抗病毒先天免疫的确切影响在很大程度上仍然难以捉摸。在这里,通过筛选代谢物库,棕榈酸 (PA) 已被确定为人体细胞中抗病毒感染的关键调节剂。从机制上讲,PA 诱导线粒体抗病毒信号蛋白 (MAVS) 棕榈酰化、聚集和随后的激活,从而增强先天免疫反应。棕榈酰转移酶ZDHHC24催化 MAVS 棕榈酰化,从而增强 TBK1-IRF3-干扰素 (IFN) 通路,特别是在 PA 刺激或高脂肪饮食喂养小鼠模型的条件下,导致抗病毒免疫反应。此外,APT2 去棕榈酰化 MAVS,从而抑制抗病毒信号传导,表明其抑制剂(如 ML349)可有效逆转 MAVS 激活以响应抗病毒感染。这些发现强调了 PA 在通过 MAVS 棕榈酰化调节抗病毒先天免疫中的关键作用,并为增加 PA 摄入量或靶向 APT2 以对抗病毒感染提供了策略。
更新日期:2024-09-09
中文翻译:

靶向 APT2 可改善 MAVS 棕榈酰化和抗病毒先天免疫
先天免疫是人类抵御病毒和微生物感染的主要防御措施。细胞代谢物,尤其是脂肪酸,对抗病毒先天免疫的确切影响在很大程度上仍然难以捉摸。在这里,通过筛选代谢物库,棕榈酸 (PA) 已被确定为人体细胞中抗病毒感染的关键调节剂。从机制上讲,PA 诱导线粒体抗病毒信号蛋白 (MAVS) 棕榈酰化、聚集和随后的激活,从而增强先天免疫反应。棕榈酰转移酶ZDHHC24催化 MAVS 棕榈酰化,从而增强 TBK1-IRF3-干扰素 (IFN) 通路,特别是在 PA 刺激或高脂肪饮食喂养小鼠模型的条件下,导致抗病毒免疫反应。此外,APT2 去棕榈酰化 MAVS,从而抑制抗病毒信号传导,表明其抑制剂(如 ML349)可有效逆转 MAVS 激活以响应抗病毒感染。这些发现强调了 PA 在通过 MAVS 棕榈酰化调节抗病毒先天免疫中的关键作用,并为增加 PA 摄入量或靶向 APT2 以对抗病毒感染提供了策略。































 京公网安备 11010802027423号
京公网安备 11010802027423号