当前位置:
X-MOL 学术
›
Cancer Cell
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Fibrotic response to anti-CSF-1R therapy potentiates glioblastoma recurrence
Cancer Cell ( IF 48.8 ) Pub Date : 2024-09-09 , DOI: 10.1016/j.ccell.2024.08.012 Spencer S Watson 1 , Anoek Zomer 2 , Nadine Fournier 3 , Joao Lourenco 3 , Manfredo Quadroni 4 , Agnieszka Chryplewicz 5 , Sina Nassiri 3 , Pauline Aubel 1 , Simona Avanthay 2 , Davide Croci 2 , Erik Abels 6 , Marike L D Broekman 6 , Douglas Hanahan 7 , Jason T Huse 8 , Roy T Daniel 9 , Monika E Hegi 10 , Krisztian Homicsko 11 , Giulia Cossu 12 , Andreas F Hottinger 13 , Johanna A Joyce 14
Cancer Cell ( IF 48.8 ) Pub Date : 2024-09-09 , DOI: 10.1016/j.ccell.2024.08.012 Spencer S Watson 1 , Anoek Zomer 2 , Nadine Fournier 3 , Joao Lourenco 3 , Manfredo Quadroni 4 , Agnieszka Chryplewicz 5 , Sina Nassiri 3 , Pauline Aubel 1 , Simona Avanthay 2 , Davide Croci 2 , Erik Abels 6 , Marike L D Broekman 6 , Douglas Hanahan 7 , Jason T Huse 8 , Roy T Daniel 9 , Monika E Hegi 10 , Krisztian Homicsko 11 , Giulia Cossu 12 , Andreas F Hottinger 13 , Johanna A Joyce 14
Affiliation
|
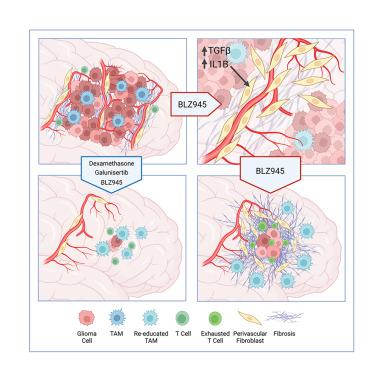
Glioblastoma recurrence is currently inevitable despite extensive standard-of-care treatment. In preclinical studies, an alternative strategy of targeting tumor-associated macrophages and microglia through CSF-1R inhibition was previously found to regress established tumors and significantly increase overall survival. However, recurrences developed in ∼50% of mice in long-term studies, which were consistently associated with fibrotic scars. This fibrotic response is observed following multiple anti-glioma therapies in different preclinical models herein and in patient recurrence samples. Multi-omics analyses of the post-treatment tumor microenvironment identified fibrotic areas as pro-tumor survival niches that encapsulated surviving glioma cells, promoted dormancy, and inhibited immune surveillance. The fibrotic treatment response was mediated by perivascular-derived fibroblast-like cells via activation by transforming growth factor β (TGF-β) signaling and neuroinflammation. Concordantly, combinatorial inhibition of these pathways inhibited treatment-associated fibrosis, and significantly improved survival in preclinical trials of anti-colony-stimulating factor-1 receptor (CSF-1R) therapy.
中文翻译:

抗 CSF-1R 治疗的纤维化反应会增强胶质母细胞瘤复发
尽管进行了广泛的标准护理治疗,但胶质母细胞瘤的复发目前是不可避免的。在临床前研究中,之前发现通过抑制 CSF-1R 靶向肿瘤相关巨噬细胞和小胶质细胞的替代策略可以使已形成的肿瘤消退并显着提高总体生存率。然而,在长期研究中,约 50% 的小鼠出现复发,这始终与纤维化疤痕相关。在本文的不同临床前模型和患者复发样本中进行多种抗神经胶质瘤治疗后观察到这种纤维化反应。对治疗后肿瘤微环境的多组学分析发现,纤维化区域是促肿瘤存活的微环境,它包裹着存活的神经胶质瘤细胞,促进休眠并抑制免疫监视。纤维化治疗反应是由血管周围来源的成纤维细胞样细胞通过转化生长因子 β (TGF-β) 信号传导和神经炎症激活来介导的。一致地,在抗集落刺激因子 1 受体 (CSF-1R) 治疗的临床前试验中,这些途径的组合抑制可抑制治疗相关的纤维化,并显着提高生存率。
更新日期:2024-09-09
中文翻译:

抗 CSF-1R 治疗的纤维化反应会增强胶质母细胞瘤复发
尽管进行了广泛的标准护理治疗,但胶质母细胞瘤的复发目前是不可避免的。在临床前研究中,之前发现通过抑制 CSF-1R 靶向肿瘤相关巨噬细胞和小胶质细胞的替代策略可以使已形成的肿瘤消退并显着提高总体生存率。然而,在长期研究中,约 50% 的小鼠出现复发,这始终与纤维化疤痕相关。在本文的不同临床前模型和患者复发样本中进行多种抗神经胶质瘤治疗后观察到这种纤维化反应。对治疗后肿瘤微环境的多组学分析发现,纤维化区域是促肿瘤存活的微环境,它包裹着存活的神经胶质瘤细胞,促进休眠并抑制免疫监视。纤维化治疗反应是由血管周围来源的成纤维细胞样细胞通过转化生长因子 β (TGF-β) 信号传导和神经炎症激活来介导的。一致地,在抗集落刺激因子 1 受体 (CSF-1R) 治疗的临床前试验中,这些途径的组合抑制可抑制治疗相关的纤维化,并显着提高生存率。































 京公网安备 11010802027423号
京公网安备 11010802027423号