当前位置:
X-MOL 学术
›
Biotechnol. Bioeng.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Auto-transduction in lentiviral vector bioprocessing: A quantitative assessment and a novel inhibition strategy
Biotechnology and Bioengineering ( IF 3.5 ) Pub Date : 2024-09-08 , DOI: 10.1002/bit.28834 Thomas Williams-Fegredo 1, 2 , Lee Davies 1 , Carol Knevelman 1 , James Miskin 1 , Kyriacos Mitrophanous 1 , Qasim A Rafiq 2
Biotechnology and Bioengineering ( IF 3.5 ) Pub Date : 2024-09-08 , DOI: 10.1002/bit.28834 Thomas Williams-Fegredo 1, 2 , Lee Davies 1 , Carol Knevelman 1 , James Miskin 1 , Kyriacos Mitrophanous 1 , Qasim A Rafiq 2
Affiliation
|
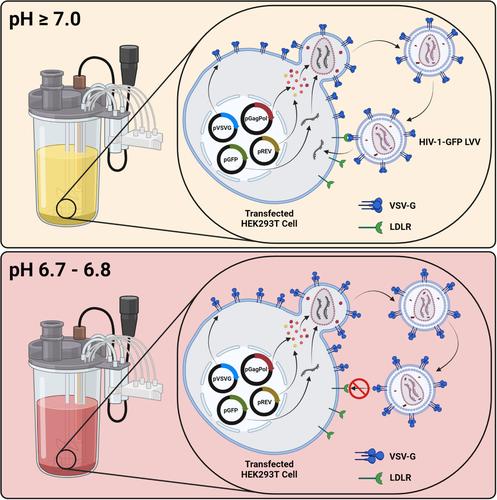
Lentiviral vectors are highly efficient gene delivery vehicles used extensively in the rapidly growing field of cell and gene therapy. Demand for efficient, large-scale, lentiviral vector bioprocessing is growing as more therapies reach late-stage clinical trials and are commercialized. However, despite substantial progress, several process inefficiencies remain. The unintended auto-transduction of viral vector-producing cells by newly synthesized lentiviral vector particles during manufacturing processes constitutes one such inefficiency which remains largely unaddressed. In this study, we determined that over 60% of functional lentiviral vector particles produced during an upstream production process were lost to auto-transduction, highlighting a major process inefficiency likely widespread within the industry. Auto-transduction of cells by particles pseudotyped with the widely used vesicular stomatitis virus G protein was inhibited via the adoption of a reduced extracellular pH during vector production, impairing the ability of the vector to interact with its target receptor. Employing a posttransfection pH shift to pH 6.7–6.8 resulted in a sevenfold reduction in vector genome integration events, arising from lentiviral vector-mediated transduction, within viral vector-producing cell populations and ultimately resulted in improved lentiviral vector production kinetics. The proposed strategy is scalable and cost-effective, providing an industrially relevant approach to improve lentiviral vector production efficiencies.
中文翻译:

慢病毒载体生物加工中的自转导:定量评估和新的抑制策略
慢病毒载体是高效基因递送载体,广泛用于快速增长的细胞和基因治疗领域。随着越来越多的疗法进入后期临床试验并商业化,对高效、大规模的慢病毒载体生物工艺的需求正在增长。然而,尽管取得了重大进展,但仍存在一些流程效率低下的问题。在制造过程中,新合成的慢病毒载体颗粒意外地自动转导产生病毒载体的细胞构成了一种在很大程度上仍未解决的低效率问题。在这项研究中,我们确定在上游生产过程中产生的功能性慢病毒载体颗粒中,超过 60% 因自转导而丢失,这凸显了行业内可能普遍存在的主要工艺效率低下。在载体生产过程中,通过采用降低的细胞外 pH 值,抑制了用广泛使用的水泡性口炎病毒 G 蛋白假型的颗粒对细胞的自转导,从而损害了载体与其靶受体相互作用的能力。转染后 pH 值转换至 pH 值 6.7–6.8 导致病毒载体生产细胞群中由慢病毒载体介导的转导引起的载体基因组整合事件减少 7 倍,并最终导致慢病毒载体生产动力学的改善。拟议的策略具有可扩展性和成本效益,提供了一种与行业相关的方法来提高慢病毒载体的生产效率。
更新日期:2024-09-08
中文翻译:

慢病毒载体生物加工中的自转导:定量评估和新的抑制策略
慢病毒载体是高效基因递送载体,广泛用于快速增长的细胞和基因治疗领域。随着越来越多的疗法进入后期临床试验并商业化,对高效、大规模的慢病毒载体生物工艺的需求正在增长。然而,尽管取得了重大进展,但仍存在一些流程效率低下的问题。在制造过程中,新合成的慢病毒载体颗粒意外地自动转导产生病毒载体的细胞构成了一种在很大程度上仍未解决的低效率问题。在这项研究中,我们确定在上游生产过程中产生的功能性慢病毒载体颗粒中,超过 60% 因自转导而丢失,这凸显了行业内可能普遍存在的主要工艺效率低下。在载体生产过程中,通过采用降低的细胞外 pH 值,抑制了用广泛使用的水泡性口炎病毒 G 蛋白假型的颗粒对细胞的自转导,从而损害了载体与其靶受体相互作用的能力。转染后 pH 值转换至 pH 值 6.7–6.8 导致病毒载体生产细胞群中由慢病毒载体介导的转导引起的载体基因组整合事件减少 7 倍,并最终导致慢病毒载体生产动力学的改善。拟议的策略具有可扩展性和成本效益,提供了一种与行业相关的方法来提高慢病毒载体的生产效率。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号