当前位置:
X-MOL 学术
›
Chem. Eng. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Fick and Maxwell-Stefan diffusion of the liquid mixture cyclohexane + toluene + acetone + methanol and its subsystems
Chemical Engineering Science ( IF 4.1 ) Pub Date : 2024-09-03 , DOI: 10.1016/j.ces.2024.120662 Yuqi Su , Denis Saric , Gabriela Guevara-Carrion , Ying Zhang , Maogang He , Jadran Vrabec
Chemical Engineering Science ( IF 4.1 ) Pub Date : 2024-09-03 , DOI: 10.1016/j.ces.2024.120662 Yuqi Su , Denis Saric , Gabriela Guevara-Carrion , Ying Zhang , Maogang He , Jadran Vrabec
|
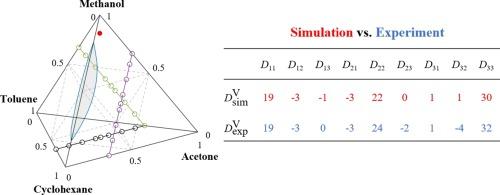
Transport diffusion of the quaternary mixture cyclohexane + toluene + acetone + methanol and most of its binary and ternary subsystems at ambient conditions of temperature and pressure is studied by molecular dynamics simulation. Liquid-liquid phase separation of cyclohexane + methanol extends into the according ternary and quaternary mixtures, rendering them highly non-ideal. Maxwell-Stefan diffusion and intradiffusion coefficients are predicted with the Green-Kubo formalism, while the thermodynamic factor is sampled with Kirkwood-Buff integration. From these data, the Fick diffusion coefficient is determined and thoroughly compared with experimental literature values. Given that the simulation results for all binaries, one of the ternaries and the quaternary mixture are successfully validated, it can be assumed that the results for the three remaining ternary mixtures, for which no experimental data exist, are sound, too. These three ternaries exhibit non-idealities that entail Maxwell-Stefan diffusion coefficient maxima that are accompanied by Fick diffusion coefficient minima due to the thermodynamic factor. Particularly the very different nature of cyclohexane and methanol molecules, where the latter strongly self-associate, leads to anomalies. The predictive models by Li et al. (2001) and Allie-Ebrahim et al. (2018) for binary and ternary mixtures, respectively, are found to yield good results for predicting the Fick diffusion coefficient of these challenging systems.
中文翻译:

液体混合物环己烷 + 甲苯 + 丙酮 + 甲醇及其子系统的 Fick 和 Maxwell-Stefan 扩散
通过分子动力学模拟研究了季元混合物环己烷 + 甲苯 + 丙酮 + 甲醇及其大多数二元和三元子系统在温度和压力环境条件下的传输扩散。环己烷 + 甲醇的液-液相分离延伸到相应的三元和四元混合物中,使它们非常不理想。Maxwell-Stefan 扩散系数和内部扩散系数使用 Green-Kubo 形式预测,而热力学因子使用 Kirkwood-Buff 积分进行采样。根据这些数据,确定 Fick 扩散系数并与实验文献值进行彻底比较。鉴于所有二元、一个三元和四元混合物的模拟结果都已成功验证,可以假设其余三个三元混合物(没有实验数据)的结果也是合理的。这三个三元表现出非理想性,这些非理想性需要 Maxwell-Stefan 扩散系数最大值,由于热力学因子,该最大值伴随着 Fick 扩散系数最小值。特别是环己烷和甲醇分子的性质截然不同,后者具有很强的自缔合性,这导致了异常。Li et al. (2001) 和 Allie-Ebrahim et al. (2018) 分别针对二元和三元混合物的预测模型被发现在预测这些具有挑战性系统的 Fick 扩散系数方面产生了很好的结果。
更新日期:2024-09-03
中文翻译:

液体混合物环己烷 + 甲苯 + 丙酮 + 甲醇及其子系统的 Fick 和 Maxwell-Stefan 扩散
通过分子动力学模拟研究了季元混合物环己烷 + 甲苯 + 丙酮 + 甲醇及其大多数二元和三元子系统在温度和压力环境条件下的传输扩散。环己烷 + 甲醇的液-液相分离延伸到相应的三元和四元混合物中,使它们非常不理想。Maxwell-Stefan 扩散系数和内部扩散系数使用 Green-Kubo 形式预测,而热力学因子使用 Kirkwood-Buff 积分进行采样。根据这些数据,确定 Fick 扩散系数并与实验文献值进行彻底比较。鉴于所有二元、一个三元和四元混合物的模拟结果都已成功验证,可以假设其余三个三元混合物(没有实验数据)的结果也是合理的。这三个三元表现出非理想性,这些非理想性需要 Maxwell-Stefan 扩散系数最大值,由于热力学因子,该最大值伴随着 Fick 扩散系数最小值。特别是环己烷和甲醇分子的性质截然不同,后者具有很强的自缔合性,这导致了异常。Li et al. (2001) 和 Allie-Ebrahim et al. (2018) 分别针对二元和三元混合物的预测模型被发现在预测这些具有挑战性系统的 Fick 扩散系数方面产生了很好的结果。

































 京公网安备 11010802027423号
京公网安备 11010802027423号