当前位置:
X-MOL 学术
›
Tetrahedron
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
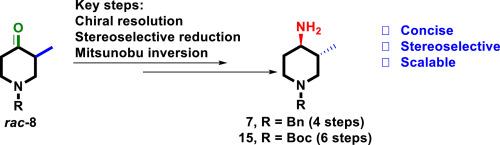
A concise, stereoselective and scalable synthesis of optically pure (3R,4R)-1-benzyl- and (3R,4R)-1-Boc-3-methyl-4-aminopiperidines
Tetrahedron ( IF 2.1 ) Pub Date : 2024-09-02 , DOI: 10.1016/j.tet.2024.134239 Sunit Hazra , Ankit Kangune , Govind Pawar , Ananta Karmakar , Kumar B. Pabbisetty , Deborah S. Mortensen , Dharmpal S. Dodd , Jianqing Li , Arvind Mathur , Anuradha Gupta , Roshan Y. Nimje
Tetrahedron ( IF 2.1 ) Pub Date : 2024-09-02 , DOI: 10.1016/j.tet.2024.134239 Sunit Hazra , Ankit Kangune , Govind Pawar , Ananta Karmakar , Kumar B. Pabbisetty , Deborah S. Mortensen , Dharmpal S. Dodd , Jianqing Li , Arvind Mathur , Anuradha Gupta , Roshan Y. Nimje
|
An efficient, scalable and stereoselective synthesis of optically pure (3R,4R )-1-benzyl- and (3R,4R )-1-Boc-3-methyl-4-aminopiperidines has been developed starting from commercially available N -benzyl-3-methyl-4-piperidone. The synthesis employed chiral resolution of N -benzyl-3-methyl-4-piperidone, cis -selective reduction of a keto group, and subsequent Mitsunobu inversion of the resulting hydroxy group to install an amine with the desired trans -stereochemistry as the key steps. The method described herein demonstrated a competent route to the title compounds in a cost-effective, and scalable manner.
中文翻译:

一种简明、立体选择性和可扩展的光学纯 (3R,4R)-1-苄基-和 (3R,4R)-1-Boc-3-甲基-4-氨基哌啶的合成
从市售的 N-苄基-3-甲基-4-哌啶开始,开发了一种高效、可扩展和立体选择性的光学纯 (3R,4R)-1-苄基-1-苄基-和 (3R,4R)-1-Boc-3-甲基-4-氨基哌啶的合成。合成采用 N-苄基-3-甲基-4-哌啶酮的手性分离、酮基的顺式选择性还原以及随后所得羟基的 Mitsunobu 反转,以安装具有所需跨立体化学的胺作为关键步骤。本文描述的方法以经济高效且可扩展的方式证明了获得标题化合物的有效途径。
更新日期:2024-09-02
中文翻译:

一种简明、立体选择性和可扩展的光学纯 (3R,4R)-1-苄基-和 (3R,4R)-1-Boc-3-甲基-4-氨基哌啶的合成
从市售的 N-苄基-3-甲基-4-哌啶开始,开发了一种高效、可扩展和立体选择性的光学纯 (3R,4R)-1-苄基-1-苄基-和 (3R,4R)-1-Boc-3-甲基-4-氨基哌啶的合成。合成采用 N-苄基-3-甲基-4-哌啶酮的手性分离、酮基的顺式选择性还原以及随后所得羟基的 Mitsunobu 反转,以安装具有所需跨立体化学的胺作为关键步骤。本文描述的方法以经济高效且可扩展的方式证明了获得标题化合物的有效途径。






























 京公网安备 11010802027423号
京公网安备 11010802027423号