当前位置:
X-MOL 学术
›
J. Hazard. Mater.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Construction of montmorillonite-based materials for highly efficient uranium removal: adsorption behaviors and mechanism
Journal of Hazardous Materials ( IF 12.2 ) Pub Date : 2024-09-05 , DOI: 10.1016/j.jhazmat.2024.135741 Jun Liao 1 , CongCong Ding 1 , Liang Jiang 1 , Junping Shi 1 , Qiuyi Wang 1 , Zihao Wang 1 , Lielin Wang 1
Journal of Hazardous Materials ( IF 12.2 ) Pub Date : 2024-09-05 , DOI: 10.1016/j.jhazmat.2024.135741 Jun Liao 1 , CongCong Ding 1 , Liang Jiang 1 , Junping Shi 1 , Qiuyi Wang 1 , Zihao Wang 1 , Lielin Wang 1
Affiliation
|
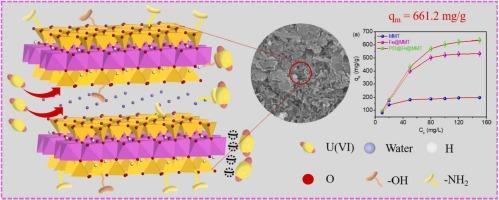
In this work, Fe3+ -doped and -NH2 -grafted montmorillonite-based material was prepared and the adsorption ability for uranium(VI) was verified. The microstructure and pore size distribution of the montmorillonite-based material were investigated by N2 adsorption-desorption analyzer and scanning electron microscopy. The surface groups and composition were analyzed by Fourier transform infrared spectrometer, X-ray photoelectron spectrometer and X-ray diffractometer, which proved the successful doping of Fe3+ and grafting of -NH2 . In the adsorption study, the adsorption reached equilibrium within 100 min with a maximum adsorption capacity of 661.2 mg/g at pH = 6 and a high adsorption efficiency of 99.4 % at low uranium(VI) concentration (pH = 6, m/V = 0.5 g/L). The mechanism study showed that the strong synergistic complexation of -OH and -NH2 for uranium(VI) played a decisive role in the adsorption process and the transport function of interlayer bound water could also enhance the adsorption probability of uranium(VI) species. These results were far superior to other reported similar materials, which proved that the Fe3+ -doped and -NH2 -grafted montmorillonite-based material possessed an extremely high application potential in adsorption, providing a new route for the modification of montmorillonite.
中文翻译:

用于高效脱铀的蒙脱石基材料的构建:吸附行为和机理
本工作制备了 Fe3+ 掺杂和 -NH2 接枝蒙脱土基材料,验证了对铀 (VI) 的吸附能力。通过 N2 吸附脱附分析仪和扫描电子显微镜研究了蒙脱石基材料的微观结构和孔径分布。通过傅里叶变换红外光谱仪、X 射线光电子能谱仪和 X 射线衍射仪分析了表面基团和组成,证明了 Fe3+ 的成功掺杂和 -NH2 的接枝。在吸附研究中,吸附在 100 分钟内达到平衡,在 pH = 6 时最大吸附容量为 661.2 mg/g,在低铀 (VI) 浓度 (pH = 6, m/V = 0.5 g/L) 下吸附效率高达 99.4 %。机理研究表明,-OH 和 -NH2 对铀 (VI) 的强协同络合在吸附过程中起决定性作用,层间结合水的传输功能也可以增强铀 (VI) 物种的吸附概率。这些结果远优于其他已报道的类似材料,证明 Fe3+ 掺杂和 -NH2 接枝蒙脱土基材料在吸附方面具有极高的应用潜力,为蒙脱石的改性提供了新的途径。
更新日期:2024-09-05
中文翻译:

用于高效脱铀的蒙脱石基材料的构建:吸附行为和机理
本工作制备了 Fe3+ 掺杂和 -NH2 接枝蒙脱土基材料,验证了对铀 (VI) 的吸附能力。通过 N2 吸附脱附分析仪和扫描电子显微镜研究了蒙脱石基材料的微观结构和孔径分布。通过傅里叶变换红外光谱仪、X 射线光电子能谱仪和 X 射线衍射仪分析了表面基团和组成,证明了 Fe3+ 的成功掺杂和 -NH2 的接枝。在吸附研究中,吸附在 100 分钟内达到平衡,在 pH = 6 时最大吸附容量为 661.2 mg/g,在低铀 (VI) 浓度 (pH = 6, m/V = 0.5 g/L) 下吸附效率高达 99.4 %。机理研究表明,-OH 和 -NH2 对铀 (VI) 的强协同络合在吸附过程中起决定性作用,层间结合水的传输功能也可以增强铀 (VI) 物种的吸附概率。这些结果远优于其他已报道的类似材料,证明 Fe3+ 掺杂和 -NH2 接枝蒙脱土基材料在吸附方面具有极高的应用潜力,为蒙脱石的改性提供了新的途径。































 京公网安备 11010802027423号
京公网安备 11010802027423号