当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Enantioselective Synthesis of Cyclopentanes by Phosphine-Catalyzed β,γ-Annulation of Allenoates
Organic Letters ( IF 4.9 ) Pub Date : 2024-09-06 , DOI: 10.1021/acs.orglett.4c02371 Chenxi Zhang 1 , Jeremy T Maddigan-Wyatt 1 , Xuan Nguyen 1 , Antonia Seitz 1 , Martin Breugst 2 , David W Lupton 1
Organic Letters ( IF 4.9 ) Pub Date : 2024-09-06 , DOI: 10.1021/acs.orglett.4c02371 Chenxi Zhang 1 , Jeremy T Maddigan-Wyatt 1 , Xuan Nguyen 1 , Antonia Seitz 1 , Martin Breugst 2 , David W Lupton 1
Affiliation
|
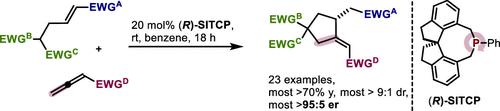
Herein, we report the enantioselective phosphine-catalyzed β,γ-annulation of electron-poor allenes with bifunctional malonates. The reaction exploits a 2C phosphonium synthon that when accessed using (R)-SITCP gives 23 cyclopentanes with high stereoselectivity (most >95:5 er and >9:1 dr) and yield. In addition to the (3+2) annulation, a one-pot three-component variant to give the same cyclopentanes and a (3+2) annulation/Dieckmann cyclization cascade, along with mechanistic studies, are reported.
中文翻译:

膦催化联烯酸酯 β,γ-环化对映选择性合成环戊烷
在此,我们报道了缺电子丙二烯与双功能丙二酸酯的对映选择性膦催化β,γ-成环反应。该反应利用 2C 鏻合成子,当使用 ( R )-SITCP 访问时,可产生具有高立体选择性(大多数 >95:5 er 和 >9:1 dr)和产率的 23 种环戊烷。除了 (3+2) 环化之外,还报道了产生相同环戊烷的一锅三组分变体和 (3+2) 环化/迪克曼环化级联以及机理研究。
更新日期:2024-09-06
中文翻译:

膦催化联烯酸酯 β,γ-环化对映选择性合成环戊烷
在此,我们报道了缺电子丙二烯与双功能丙二酸酯的对映选择性膦催化β,γ-成环反应。该反应利用 2C 鏻合成子,当使用 ( R )-SITCP 访问时,可产生具有高立体选择性(大多数 >95:5 er 和 >9:1 dr)和产率的 23 种环戊烷。除了 (3+2) 环化之外,还报道了产生相同环戊烷的一锅三组分变体和 (3+2) 环化/迪克曼环化级联以及机理研究。































 京公网安备 11010802027423号
京公网安备 11010802027423号