当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Rhodium-Catalyzed Double Dearomatization of 1,2,3-Triazole–Isoxazole Dyads: Synthesis of Nonfused 1H-1,3-Diazepines
Organic Letters ( IF 4.9 ) Pub Date : 2024-09-06 , DOI: 10.1021/acs.orglett.4c02588 Gleb D Titov 1 , Alexander S Bunev 2 , Svetlana V Urusova 1 , Mikhail S Novikov 1 , Alexander F Khlebnikov 1 , Nikolai V Rostovskii 1
Organic Letters ( IF 4.9 ) Pub Date : 2024-09-06 , DOI: 10.1021/acs.orglett.4c02588 Gleb D Titov 1 , Alexander S Bunev 2 , Svetlana V Urusova 1 , Mikhail S Novikov 1 , Alexander F Khlebnikov 1 , Nikolai V Rostovskii 1
Affiliation
|
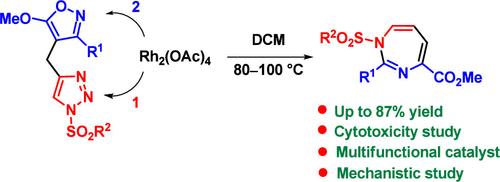
A double dearomatization of dyads consisting of 1-sulfonyl-1,2,3-triazoles and 3-aryl-5-methoxyisoxazoles was applied for the efficient synthesis of nonfused 1H-1,3-diazepines. The plausible mechanism of the cascade reaction includes transformation of the 1,2,3-triazole to rhodium azavinyl carbene, the Z-selective hydride shift to form the 1-azabuta-1,3-diene moiety, rhodium-catalyzed ring contraction of the isoxazole to azirine, and pseudopericyclic four-atom ring expansion of the azirine. The synthetic utility and antiproliferative activity of the 1,3-diazepines obtained have been demonstrated.
中文翻译:

铑催化 1,2,3-三唑-异恶唑二元组的双重脱芳构化:非稠合 1H-1,3-二氮杂卓的合成
由1-磺酰基-1,2,3-三唑和3-芳基-5-甲氧基异恶唑组成的二元体的双重脱芳构化被应用于非稠合1 H -1,3-二氮杂卓的有效合成。级联反应的合理机制包括 1,2,3-三唑转化为铑氮杂乙烯基卡宾, Z-选择性氢化物位移形成 1-azabuta-1,3-二烯部分,铑催化的环收缩异恶唑变为氮丙啶,以及氮丙啶的假周环四原子环扩展。所获得的1,3-二氮杂卓的合成用途和抗增殖活性已得到证实。
更新日期:2024-09-06
中文翻译:

铑催化 1,2,3-三唑-异恶唑二元组的双重脱芳构化:非稠合 1H-1,3-二氮杂卓的合成
由1-磺酰基-1,2,3-三唑和3-芳基-5-甲氧基异恶唑组成的二元体的双重脱芳构化被应用于非稠合1 H -1,3-二氮杂卓的有效合成。级联反应的合理机制包括 1,2,3-三唑转化为铑氮杂乙烯基卡宾, Z-选择性氢化物位移形成 1-azabuta-1,3-二烯部分,铑催化的环收缩异恶唑变为氮丙啶,以及氮丙啶的假周环四原子环扩展。所获得的1,3-二氮杂卓的合成用途和抗增殖活性已得到证实。































 京公网安备 11010802027423号
京公网安备 11010802027423号