当前位置:
X-MOL 学术
›
Org. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Tunable Rh(III)-Catalyzed C(sp2)–H Bond Functionalization of Aryl Imidates with Cyclic 1,3-Diones: Strategic Use of Directing Groups
Organic Letters ( IF 4.9 ) Pub Date : 2024-09-06 , DOI: 10.1021/acs.orglett.4c02819 Junnan E 1 , Luohe Wang 1 , Jing Zeng 1 , Hua Tian 2 , Xiubin Bu 1 , Xiaobo Yang 1, 3 , Zhen Zhao 1
Organic Letters ( IF 4.9 ) Pub Date : 2024-09-06 , DOI: 10.1021/acs.orglett.4c02819 Junnan E 1 , Luohe Wang 1 , Jing Zeng 1 , Hua Tian 2 , Xiubin Bu 1 , Xiaobo Yang 1, 3 , Zhen Zhao 1
Affiliation
|
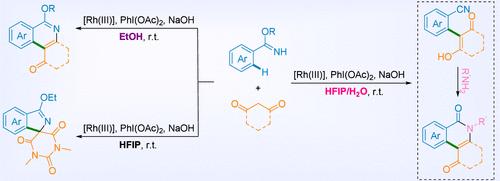
A tunable Rh(III)-catalyzed C(sp2)–H bond functionalization of aryl imidates with cyclic 1,3-diones was developed. With suitable and straightforward reaction condition adjustments, the C–H bond functionalization of diverse aryl imidates with cyclic 1,3-diones occurred smoothly and precisely at room temperature. Accompanied by different directing group transformations, a series of corresponding aryl nitriles, hydrophenanthridin-1(2H)-ones, spiro isoindoles, or hydrophenanthridine-1,6(2H,5H)-diones were synthesized in good yields to provide a rational directing group utilization strategy for the Rh(III)-catalyzed C(sp2)–H bond activation. Control experiments and primary mechanistic studies revealed that solvent effects and functional group electronic effects might influence the reaction’s selectivity.
中文翻译:

环状 1,3-二酮对芳基亚胺酸酯的可调 Rh(III) 催化 C(sp2)–H 键官能化:定向基团的战略使用
开发了一种可调节的 Rh(III) 催化的亚胺酸芳基酯与环状 1,3-二酮的 C(sp 2 )-H 键官能化。通过适当且直接的反应条件调整,各种芳基亚胺酸酯与环状1,3-二酮的C-H键官能化在室温下顺利且精确地发生。伴随不同的导向基团转化,以良好的收率合成了一系列相应的芳基腈、氢菲啶-1(2 H )-酮、螺异吲哚或氢菲啶-1,6(2 H ,5 H )-二酮,提供了Rh(III) 催化的 C(sp 2 )–H 键活化的合理导向基团利用策略。对照实验和主要机理研究表明,溶剂效应和官能团电子效应可能会影响反应的选择性。
更新日期:2024-09-06
中文翻译:

环状 1,3-二酮对芳基亚胺酸酯的可调 Rh(III) 催化 C(sp2)–H 键官能化:定向基团的战略使用
开发了一种可调节的 Rh(III) 催化的亚胺酸芳基酯与环状 1,3-二酮的 C(sp 2 )-H 键官能化。通过适当且直接的反应条件调整,各种芳基亚胺酸酯与环状1,3-二酮的C-H键官能化在室温下顺利且精确地发生。伴随不同的导向基团转化,以良好的收率合成了一系列相应的芳基腈、氢菲啶-1(2 H )-酮、螺异吲哚或氢菲啶-1,6(2 H ,5 H )-二酮,提供了Rh(III) 催化的 C(sp 2 )–H 键活化的合理导向基团利用策略。对照实验和主要机理研究表明,溶剂效应和官能团电子效应可能会影响反应的选择性。































 京公网安备 11010802027423号
京公网安备 11010802027423号