当前位置:
X-MOL 学术
›
J. Comput. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
A computational mechanistic study on the formation of aryl sulfonyl fluorides via Bi(III) redox-neutral catalysis and further rational design
Journal of Computational Chemistry ( IF 3.4 ) Pub Date : 2024-09-06 , DOI: 10.1002/jcc.27501 Zhaoyin Zhang 1 , Qin Ma 1 , Xing Yang 1 , Shuqi Zhang 1 , Kai Guo 2 , Lili Zhao 1
Journal of Computational Chemistry ( IF 3.4 ) Pub Date : 2024-09-06 , DOI: 10.1002/jcc.27501 Zhaoyin Zhang 1 , Qin Ma 1 , Xing Yang 1 , Shuqi Zhang 1 , Kai Guo 2 , Lili Zhao 1
Affiliation
|
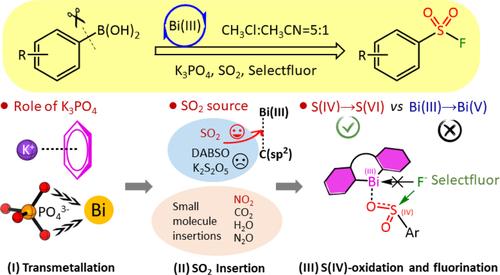
Sulfonyl fluorides hold significant importance as highly valued intermediates in chemical biology due to their optimal balance of biocompatibility with both aqueous stability and protein reactivity. The Cornella group introduced a one-pot strategy for synthesizing aryl sulfonyl fluorides via Bi(III) redox-neutral catalysis, which facilitates the transmetallation and direct insertion of SO2 into the BiC(sp2) bond giving the aryl sulfonyl fluorides. We report herein a comprehensive computational investigation of the redox-neutral Bi(III) catalytic mechanism, disclose the critical role of the Bi(III) catalyst and base (i.e., K3PO4), and uncover the origin of SO2 insertion into the Bi(III)C(sp2) bond. The entire catalysis can be characterized via three stages: (i) transmetallation generating the Bi(III)-phenyl intermediate IM3 facilitated by K3PO4. (ii) SO2 insertion into IM3 leading to the formation of Bi(III)-OSOAr intermediate IM5. (iii) IM5 undergoes S(IV)-oxidation yielding the aryl sulfonyl fluoride product 4 and liberating the Bi(III) catalyst for the next catalytic cycle. Each stage is kinetically and thermodynamically feasible. Moreover, we explored other some small molecules (NO2, CO2, H2O, N2O, etc.) insertion reactions mediated by the Bi(III)-complex, and found that NO2 insertions could be easily achieved due to the low insertion barriers (i.e., 17.5 kcal/mol). Based on the detailed mechanistic study, we further rationally designed additional Bi(III) and Sb(III) catalysts, and found that some of which exhibit promising potential for experimental realization due to their low barriers (<16.4 kcal/mol). In this regard, our study contributes significantly to enhancing current Bi(III)-catalytic systems and paving the way for novel Bi(III)-catalyzed aryl sulfonyl fluoride formation reactions.
中文翻译:

Bi(III) 氧化还原中性催化形成芳基磺酰氟的计算机理研究及进一步合理设计
磺酰氟作为化学生物学中高度有价值的中间体具有重要意义,因为它们在生物相容性与水稳定性和蛋白质反应性之间取得了最佳平衡。Cornella 小组引入了一种通过 Bi(III) 氧化还原中性催化合成芳基磺酰氟的单锅法,这有助于 SO2 转移金属并直接插入 BiC(sp2) 键中,得到芳基磺酰氟。我们在此报告了对氧化还原中性 Bi(III) 催化机制的全面计算研究,揭示了 Bi(III) 催化剂和碱(即 K3PO4)的关键作用,并揭示了 SO2 插入 Bi(III)C(sp2) 键的起源。整个催化过程可以通过三个阶段来表征:(i) 金属转移生成由 K3PO4 促进的 Bi(III)-苯基中间体 IM3。(ii) SO2 插入 IM3 导致形成 Bi(III)-OSOAr 中间体 IM5。(iii) IM5 发生 S(IV) 氧化,产生芳基磺酰氟产物 4 并释放 Bi(III) 催化剂用于下一个催化循环。每个阶段在动力学和热力学上都是可行的。此外,我们探索了由 Bi(III) 复合物介导的其他一些小分子 (NO2、CO2、H2O、N2O 等) 插入反应,发现由于插入屏障低(即 17.5 kcal/mol),很容易实现 NO2 插入。 基于详细的机理研究,我们进一步合理地设计了额外的 Bi(III) 和 Sb(III) 催化剂,发现其中一些催化剂由于其低阻垒性 (<16.4 kcal/mol) 而表现出有希望的实验实现潜力。在这方面,我们的研究对增强当前的 Bi(III) 催化体系和为新型 Bi(III) 催化的芳基磺酰氟形成反应铺平了道路做出了重大贡献。
更新日期:2024-09-06
中文翻译:

Bi(III) 氧化还原中性催化形成芳基磺酰氟的计算机理研究及进一步合理设计
磺酰氟作为化学生物学中高度有价值的中间体具有重要意义,因为它们在生物相容性与水稳定性和蛋白质反应性之间取得了最佳平衡。Cornella 小组引入了一种通过 Bi(III) 氧化还原中性催化合成芳基磺酰氟的单锅法,这有助于 SO2 转移金属并直接插入 BiC(sp2) 键中,得到芳基磺酰氟。我们在此报告了对氧化还原中性 Bi(III) 催化机制的全面计算研究,揭示了 Bi(III) 催化剂和碱(即 K3PO4)的关键作用,并揭示了 SO2 插入 Bi(III)C(sp2) 键的起源。整个催化过程可以通过三个阶段来表征:(i) 金属转移生成由 K3PO4 促进的 Bi(III)-苯基中间体 IM3。(ii) SO2 插入 IM3 导致形成 Bi(III)-OSOAr 中间体 IM5。(iii) IM5 发生 S(IV) 氧化,产生芳基磺酰氟产物 4 并释放 Bi(III) 催化剂用于下一个催化循环。每个阶段在动力学和热力学上都是可行的。此外,我们探索了由 Bi(III) 复合物介导的其他一些小分子 (NO2、CO2、H2O、N2O 等) 插入反应,发现由于插入屏障低(即 17.5 kcal/mol),很容易实现 NO2 插入。 基于详细的机理研究,我们进一步合理地设计了额外的 Bi(III) 和 Sb(III) 催化剂,发现其中一些催化剂由于其低阻垒性 (<16.4 kcal/mol) 而表现出有希望的实验实现潜力。在这方面,我们的研究对增强当前的 Bi(III) 催化体系和为新型 Bi(III) 催化的芳基磺酰氟形成反应铺平了道路做出了重大贡献。































 京公网安备 11010802027423号
京公网安备 11010802027423号