当前位置:
X-MOL 学术
›
J. Mater. Chem. A
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
The aerosol-assisted chemical vapour deposition of Mo-doped BiVO4 photoanodes for solar water splitting: an experimental and computational study
Journal of Materials Chemistry A ( IF 10.7 ) Pub Date : 2024-09-06 , DOI: 10.1039/d4ta02605e Shaobin Zhao 1 , Chenglin Jia 2 , Xinyi Shen 1 , Ruohao Li 1 , Louise Oldham 1 , Benjamin Moss 1 , Brian Tam 1, 3 , Sebastian Pike 4 , Nicholas Harrison 1, 5 , Ehsan Ahmad 1, 5 , Andreas Kafizas 1, 6
Journal of Materials Chemistry A ( IF 10.7 ) Pub Date : 2024-09-06 , DOI: 10.1039/d4ta02605e Shaobin Zhao 1 , Chenglin Jia 2 , Xinyi Shen 1 , Ruohao Li 1 , Louise Oldham 1 , Benjamin Moss 1 , Brian Tam 1, 3 , Sebastian Pike 4 , Nicholas Harrison 1, 5 , Ehsan Ahmad 1, 5 , Andreas Kafizas 1, 6
Affiliation
|
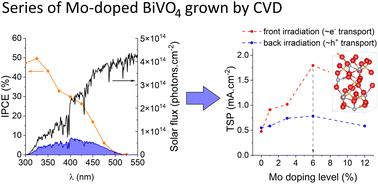
BiVO4 is one of the most promising light absorbing materials for use in photoelectrochemical (PEC) water splitting devices. Although intrinsic BiVO4 suffers from poor charge carrier mobility, this can be overcome by Mo-doping. However, for Mo-doped BiVO4 to be applied in commercial PEC water splitting devices, scalable routes to high performance materials need to be developed. Herein, we propose a scalable aerosol-assisted chemical vapour deposition (AA-CVD) route to high performance Mo-doped BiVO4. The materials were characterised using X-ray diffraction (XRD), Raman spectroscopy, X-ray photoelectron spectroscopy (XPS), scanning electron microscopy (SEM), atomic force microscopy (AFM), UV-visible absorption spectroscopy, and a range of PEC tests. By studying a range of Mo-precursor doping levels (0 to 12% Mo : V), an optimum precursor doping level was found (6% Mo : V); substituting V5+ sites in the host structure as Mo6+. In PEC water oxidation the highest performing material showed an onset of photocurrent (Jon) at ∼0.6 VRHE and a theoretical solar photocurrent (TSP) of ∼1.79 mA cm−2 at 1.23 VRHE and 1 sun irradiance. Importantly, Mo-doping was found to induce a phase change from monoclinic clinobisvanite (m-BiVO4), found in undoped BiVO4, to tetragonal scheelite (t-BiVO4). The effect of Mo-doping on the phase stability, structural and electronic properties was examined with all-electron hybrid exchange density functional theory (DFT) calculations. Doping into V and Bi sites at 6.25 and 12.5 at% was calculated for t-BiVO4 and m-BiVO4 phases. In accord with our observations, 6.25 at% Mo doping into the V sites in t-BiVO4 is found to be energetically favoured over doping into m-BiVO4 (by 2.33 meV per Mo atom inserted). The computed charge density is consistent with n-doping of the lattice as Mo6+ replaces V5+ generating an occupied mid-gap state ∼0.4 eV below the conduction band minimum (CBM) which is primarily of Mo-4d character. Doubling this doping level to 12.5 at% in t-BiVO4 resulted in the mid-gap state merging with the CBM and the formation of a degenerate semiconductor with electrons distributed over the 3d orbitals of V ions residing in the [001] plane. In conjunction with our experimental findings, this strongly suggests that it is the increased electron conductivity due to Mo doping of BiVO4 that produces a more active photoanode for water splitting, and that this maximises between 6.25 to 12.5 at% doping.
中文翻译:

用于太阳能分解水的气溶胶辅助化学气相沉积 Mo 掺杂 BiVO4 光阳极:实验和计算研究
BiVO 4是用于光电化学(PEC)水分解装置的最有前途的光吸收材料之一。尽管本征 BiVO 4 的载流子迁移率较差,但这可以通过 Mo 掺杂来克服。然而,为了将Mo掺杂BiVO 4应用于商业PEC水分解装置,需要开发高性能材料的可扩展路线。在此,我们提出了一种可扩展的气溶胶辅助化学气相沉积(AA-CVD)路线来制备高性能Mo掺杂BiVO 4 。使用 X 射线衍射 (XRD)、拉曼光谱、X 射线光电子能谱 (XPS)、扫描电子显微镜 (SEM)、原子力显微镜 (AFM)、紫外-可见吸收光谱和一系列 PEC 对材料进行了表征测试。通过研究一系列 Mo 前体掺杂水平(0 至 12% Mo : V),找到了最佳前体掺杂水平(6% Mo : V);将主体结构中的V 5+位点替换为Mo 6+ 。在PEC水氧化中,性能最高的材料在~0.6 V RHE下表现出光电流( J on ),在1.23 V RHE和1太阳辐照度下理论太阳光电流(TSP)为~1.79 mA cm -2 。 重要的是,Mo 掺杂被发现会引起从单斜二尖锰矿(m-BiVO 4 )(在未掺杂的 BiVO 4中发现)到四方白钨矿(t-BiVO 4 )的相变。通过全电子杂化交换密度泛函理论(DFT)计算检验了钼掺杂对相稳定性、结构和电子性能的影响。计算t-BiVO 4和m-BiVO 4相以6.25和12.5 at%掺杂到V和Bi位点。根据我们的观察,发现在 t-BiVO 4中的 V 位点中掺杂 6.25 at% Mo 比掺杂到 m-BiVO 4中更有利(每个插入的 Mo 原子掺杂 2.33 meV)。计算出的电荷密度与晶格的 n 掺杂一致,因为 Mo 6+取代了 V 5+ ,产生了比导带最小值 (CBM) 低约 0.4 eV 的占据中间能隙态,其主要具有 Mo-4d 特性。在 t-BiVO 4中将此掺杂水平加倍至 12.5 at%,导致中带隙态与 CBM 合并,并形成简并半导体,电子分布在位于 [001] 平面的 V 离子的 3d 轨道上。结合我们的实验结果,这有力地表明,BiVO 4的 Mo 掺杂导致电子电导率增加,从而产生了用于水分解的更活跃的光电阳极,并且这使得 6.25 至 12.5 at% 的掺杂达到最大。
更新日期:2024-09-06
中文翻译:

用于太阳能分解水的气溶胶辅助化学气相沉积 Mo 掺杂 BiVO4 光阳极:实验和计算研究
BiVO 4是用于光电化学(PEC)水分解装置的最有前途的光吸收材料之一。尽管本征 BiVO 4 的载流子迁移率较差,但这可以通过 Mo 掺杂来克服。然而,为了将Mo掺杂BiVO 4应用于商业PEC水分解装置,需要开发高性能材料的可扩展路线。在此,我们提出了一种可扩展的气溶胶辅助化学气相沉积(AA-CVD)路线来制备高性能Mo掺杂BiVO 4 。使用 X 射线衍射 (XRD)、拉曼光谱、X 射线光电子能谱 (XPS)、扫描电子显微镜 (SEM)、原子力显微镜 (AFM)、紫外-可见吸收光谱和一系列 PEC 对材料进行了表征测试。通过研究一系列 Mo 前体掺杂水平(0 至 12% Mo : V),找到了最佳前体掺杂水平(6% Mo : V);将主体结构中的V 5+位点替换为Mo 6+ 。在PEC水氧化中,性能最高的材料在~0.6 V RHE下表现出光电流( J on ),在1.23 V RHE和1太阳辐照度下理论太阳光电流(TSP)为~1.79 mA cm -2 。 重要的是,Mo 掺杂被发现会引起从单斜二尖锰矿(m-BiVO 4 )(在未掺杂的 BiVO 4中发现)到四方白钨矿(t-BiVO 4 )的相变。通过全电子杂化交换密度泛函理论(DFT)计算检验了钼掺杂对相稳定性、结构和电子性能的影响。计算t-BiVO 4和m-BiVO 4相以6.25和12.5 at%掺杂到V和Bi位点。根据我们的观察,发现在 t-BiVO 4中的 V 位点中掺杂 6.25 at% Mo 比掺杂到 m-BiVO 4中更有利(每个插入的 Mo 原子掺杂 2.33 meV)。计算出的电荷密度与晶格的 n 掺杂一致,因为 Mo 6+取代了 V 5+ ,产生了比导带最小值 (CBM) 低约 0.4 eV 的占据中间能隙态,其主要具有 Mo-4d 特性。在 t-BiVO 4中将此掺杂水平加倍至 12.5 at%,导致中带隙态与 CBM 合并,并形成简并半导体,电子分布在位于 [001] 平面的 V 离子的 3d 轨道上。结合我们的实验结果,这有力地表明,BiVO 4的 Mo 掺杂导致电子电导率增加,从而产生了用于水分解的更活跃的光电阳极,并且这使得 6.25 至 12.5 at% 的掺杂达到最大。































 京公网安备 11010802027423号
京公网安备 11010802027423号