当前位置:
X-MOL 学术
›
Chem. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
A switch strategy for the synthesis of C4-ethylamine indole and C7-aminoindoline via controllable carbon elimination
Chemical Science ( IF 7.6 ) Pub Date : 2024-09-06 , DOI: 10.1039/d4sc05111d
Bo-Sheng Zhang 1 , Bao-Jie Deng 1 , Yuan-Xin Zhi 1 , Tian-Jiao Guo 1 , Yi-Ming Wang 1 , Xue-Ya Gou 2 , Zheng-Jun Quan 1 , Xi-Cun Wang 1 , Yong-Min Liang 2
Chemical Science ( IF 7.6 ) Pub Date : 2024-09-06 , DOI: 10.1039/d4sc05111d
Bo-Sheng Zhang 1 , Bao-Jie Deng 1 , Yuan-Xin Zhi 1 , Tian-Jiao Guo 1 , Yi-Ming Wang 1 , Xue-Ya Gou 2 , Zheng-Jun Quan 1 , Xi-Cun Wang 1 , Yong-Min Liang 2
Affiliation
|
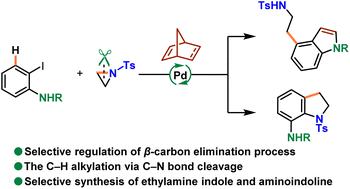
Controllable β-carbon elimination to extrude norbornene remains a long-standing challenge in palladium and norbornene chemistry. Herein, this manuscript describes a switchable synthesis of biologically active C4-ethylaminoindole and C7-aminoindoline scaffolds by controlling β-carbon elimination, utilizing aziridine as a C–H ethylamination reagent through a C–N bond cleavage reaction. Furthermore, the protecting groups of the product can be easily removed, offering an unusual method for the synthesis of dopamine receptor agonists.
中文翻译:

可控碳消除合成C4-乙胺吲哚和C7-氨基吲哚啉的切换策略
可控的β-碳消除以挤出降冰片烯仍然是钯和降冰片烯化学中长期存在的挑战。在此,该手稿描述了通过控制β-碳消除,利用氮丙啶作为C-H乙胺化试剂,通过C-N键断裂反应,可切换合成具有生物活性的C4-乙氨基吲哚和C7-氨基吲哚啉支架。此外,该产品的保护基团可以很容易地去除,为多巴胺受体激动剂的合成提供了一种不寻常的方法。
更新日期:2024-09-06
中文翻译:

可控碳消除合成C4-乙胺吲哚和C7-氨基吲哚啉的切换策略
可控的β-碳消除以挤出降冰片烯仍然是钯和降冰片烯化学中长期存在的挑战。在此,该手稿描述了通过控制β-碳消除,利用氮丙啶作为C-H乙胺化试剂,通过C-N键断裂反应,可切换合成具有生物活性的C4-乙氨基吲哚和C7-氨基吲哚啉支架。此外,该产品的保护基团可以很容易地去除,为多巴胺受体激动剂的合成提供了一种不寻常的方法。































 京公网安备 11010802027423号
京公网安备 11010802027423号