当前位置:
X-MOL 学术
›
Chem. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Enzymatic synthesis of S-adenosyl-L-homocysteine and its nucleoside analogs from racemic homocysteine thiolactone
Chemical Science ( IF 7.6 ) Pub Date : 2024-09-06 , DOI: 10.1039/d4sc03801k Xiaojin Wen 1, 2 , Viviane Leopold 1 , Florian P Seebeck 1, 2
Chemical Science ( IF 7.6 ) Pub Date : 2024-09-06 , DOI: 10.1039/d4sc03801k Xiaojin Wen 1, 2 , Viviane Leopold 1 , Florian P Seebeck 1, 2
Affiliation
|
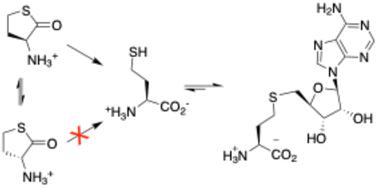
S-Adenosyl methionine (SAM)-dependent methyltransferases hold significant potential as tools for the biocatalytic synthesis of complex molecules due to their ability to methylate or alkylate substrates with high regio-, chemo-, and stereoselectivity. Recent advancements in enzyme-catalyzed S-methylation and S-alkylation of S-adenosyl homocysteine (SAH) using synthetic alkylation agents have expanded the scope of methyltransferases in preparative biocatalysis. This development has transformed SAH from an unwanted byproduct into a crucial – and currently expensive – reagent. In this report, we present a simple and scalable one-pot synthesis of SAH, starting from racemic homocysteine thiolactone and adenosine. This process is catalyzed by recombinant α-amino-ε-caprolactam racemase, bleomycin hydrolase, and SAH hydrolase. The reaction proceeds to completion with near-stoichiometric mixtures of reactants, driven by the irreversible and stereoselective hydrolysis of thiolactone, followed by the thermodynamically favorable condensation of homocysteine with adenosine. We demonstrate that this method can be utilized to supplement preparative methylation reactions with SAH as a cofactor, as well as to synthesize and screen S-nucleosyl homocysteine derivatives in the search for stabilized SAM analogs.
中文翻译:

外消旋同型半胱氨酸硫代内酯酶法合成S-腺苷-L-同型半胱氨酸及其核苷类似物
S-腺苷甲硫氨酸 (SAM) 依赖性甲基转移酶具有作为复杂分子生物催化合成工具的巨大潜力,因为它们能够以高区域选择性、化学选择性和立体选择性对底物进行甲基化或烷基化。使用合成烷基化剂对S-腺苷高半胱氨酸(SAH)进行酶催化S-甲基化和S-烷基化的最新进展扩大了甲基转移酶在制备型生物催化中的范围。这一发展已将 SAH 从一种不需要的副产品转变为一种重要且目前昂贵的试剂。在本报告中,我们提出了一种简单且可扩展的 SAH 一锅合成方法,从外消旋同型半胱氨酸硫代内酯和腺苷开始。该过程由重组 α-氨基-ε-己内酰胺消旋酶、博莱霉素水解酶和 SAH 水解酶催化。在硫内酯的不可逆和立体选择性水解的驱动下,反应以接近化学计量的反应物混合物进行,随后是热力学上有利的高半胱氨酸与腺苷的缩合。我们证明该方法可用于补充以 SAH 作为辅因子的制备甲基化反应,以及合成和筛选S-核糖基高半胱氨酸衍生物以寻找稳定的 SAM 类似物。
更新日期:2024-09-06
中文翻译:

外消旋同型半胱氨酸硫代内酯酶法合成S-腺苷-L-同型半胱氨酸及其核苷类似物
S-腺苷甲硫氨酸 (SAM) 依赖性甲基转移酶具有作为复杂分子生物催化合成工具的巨大潜力,因为它们能够以高区域选择性、化学选择性和立体选择性对底物进行甲基化或烷基化。使用合成烷基化剂对S-腺苷高半胱氨酸(SAH)进行酶催化S-甲基化和S-烷基化的最新进展扩大了甲基转移酶在制备型生物催化中的范围。这一发展已将 SAH 从一种不需要的副产品转变为一种重要且目前昂贵的试剂。在本报告中,我们提出了一种简单且可扩展的 SAH 一锅合成方法,从外消旋同型半胱氨酸硫代内酯和腺苷开始。该过程由重组 α-氨基-ε-己内酰胺消旋酶、博莱霉素水解酶和 SAH 水解酶催化。在硫内酯的不可逆和立体选择性水解的驱动下,反应以接近化学计量的反应物混合物进行,随后是热力学上有利的高半胱氨酸与腺苷的缩合。我们证明该方法可用于补充以 SAH 作为辅因子的制备甲基化反应,以及合成和筛选S-核糖基高半胱氨酸衍生物以寻找稳定的 SAM 类似物。































 京公网安备 11010802027423号
京公网安备 11010802027423号