当前位置:
X-MOL 学术
›
Anal. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Predictive Analysis in Oral Cancer Immunotherapy: Profiling Dual PD-L1-Positive Extracellular Vesicle Subtypes with Step-Wedge Microfluidic Chips
Analytical Chemistry ( IF 6.7 ) Pub Date : 2024-09-05 , DOI: 10.1021/acs.analchem.4c03101 Zi-Li Yu 1, 2 , Zhou-Yang Wu 1 , Xing-Chi Liu 1 , Chang-Xin Ji 3 , Xuan Wang 3 , Qiu-Yun Fu 1 , Gang Chen 1, 2, 4, 5 , Min Wu 1 , Shao-Li Hong 3 , Jun Jia 1, 2
Analytical Chemistry ( IF 6.7 ) Pub Date : 2024-09-05 , DOI: 10.1021/acs.analchem.4c03101 Zi-Li Yu 1, 2 , Zhou-Yang Wu 1 , Xing-Chi Liu 1 , Chang-Xin Ji 3 , Xuan Wang 3 , Qiu-Yun Fu 1 , Gang Chen 1, 2, 4, 5 , Min Wu 1 , Shao-Li Hong 3 , Jun Jia 1, 2
Affiliation
|
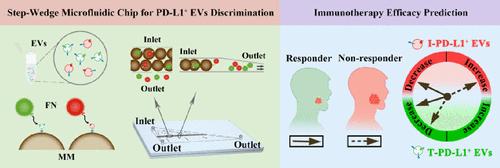
PD-L1-positive extracellular vesicles (PD-L1+ EVs) play a pivotal role as predictive biomarkers in cancer immunotherapy. These vesicles, originating from immune cells (I-PD-L1+ EVs) and tumor cells (T-PD-L1+ EVs), hold distinct clinical predictive values, emphasizing the importance of deeply differentiating the PD-L1+ EV subtypes for effective liquid biopsy analyses. However, current methods such as ELISA lack the ability to differentiate their cellular sources. In this study, a novel step-wedge microfluidic chip that combines magnetic microsphere separation with single-layer fluorescence counting is developed. This chip integrates magnetic microspheres modified with anti-PD-L1 antibodies and fluorescent nanoparticles targeting EpCAM (tumor cell marker) or CD45 (immunocyte marker), enabling simultaneous quantification and sensitive analysis of PD-L1+ EV subpopulations in oral squamous cell carcinoma (OSCC) patients’ saliva without background interference. Analysis results indicate reduced levels of I-PD-L1+ EVs in OSCC patients compared to those in healthy individuals, with varying levels of heterogeneous PD-L1+ EVs observed among different patient groups. During immunotherapy, responders exhibit decreased levels of total PD-L1+ EVs and T-PD-L1+ EVs, accompanied by reduced levels of I-PD-L1+ EVs. Conversely, nonresponders show increased levels of I-PD-L1+ EVs. Utilizing the step-wedge microfluidic chip allows for simultaneous detection of PD-L1+ EV subtypes, facilitating the precise prediction of oral cancer immunotherapy outcomes.
中文翻译:

口腔癌免疫治疗的预测分析:使用阶梯楔形微流控芯片分析双 PD-L1 阳性细胞外囊泡亚型
PD-L1 阳性细胞外囊泡 (PD-L1 + EV) 作为癌症免疫治疗中的预测生物标志物发挥着关键作用。这些囊泡源自免疫细胞(I-PD-L1 + EV)和肿瘤细胞(T-PD-L1 + EV),具有独特的临床预测价值,强调了深度区分 PD-L1 + EV 亚型对于有效治疗的重要性。液体活检分析。然而,目前的方法(例如 ELISA)缺乏区分其细胞来源的能力。在本研究中,开发了一种将磁性微球分离与单层荧光计数相结合的新型阶梯楔形微流控芯片。该芯片集成了抗PD-L1抗体修饰的磁性微球和针对EpCAM(肿瘤细胞标记物)或CD45(免疫细胞标记物)的荧光纳米粒子,能够对口腔鳞状细胞癌(OSCC)中的PD-L1 + EV亚群进行同步定量和灵敏分析)患者唾液,无背景干扰。分析结果表明,与健康个体相比,OSCC 患者的 I-PD-L1 + EV 水平降低,不同患者组之间观察到不同水平的异质 PD-L1 + EV。在免疫治疗期间,应答者表现出总 PD-L1 + EV 和 T-PD-L1 + EV 水平降低,同时 I-PD-L1 + EV 水平降低。相反,无反应者的 I-PD-L1 + EV 水平升高。利用阶梯楔形微流控芯片可以同时检测PD-L1 + EV亚型,有助于精确预测口腔癌免疫治疗结果。
更新日期:2024-09-05
中文翻译:

口腔癌免疫治疗的预测分析:使用阶梯楔形微流控芯片分析双 PD-L1 阳性细胞外囊泡亚型
PD-L1 阳性细胞外囊泡 (PD-L1 + EV) 作为癌症免疫治疗中的预测生物标志物发挥着关键作用。这些囊泡源自免疫细胞(I-PD-L1 + EV)和肿瘤细胞(T-PD-L1 + EV),具有独特的临床预测价值,强调了深度区分 PD-L1 + EV 亚型对于有效治疗的重要性。液体活检分析。然而,目前的方法(例如 ELISA)缺乏区分其细胞来源的能力。在本研究中,开发了一种将磁性微球分离与单层荧光计数相结合的新型阶梯楔形微流控芯片。该芯片集成了抗PD-L1抗体修饰的磁性微球和针对EpCAM(肿瘤细胞标记物)或CD45(免疫细胞标记物)的荧光纳米粒子,能够对口腔鳞状细胞癌(OSCC)中的PD-L1 + EV亚群进行同步定量和灵敏分析)患者唾液,无背景干扰。分析结果表明,与健康个体相比,OSCC 患者的 I-PD-L1 + EV 水平降低,不同患者组之间观察到不同水平的异质 PD-L1 + EV。在免疫治疗期间,应答者表现出总 PD-L1 + EV 和 T-PD-L1 + EV 水平降低,同时 I-PD-L1 + EV 水平降低。相反,无反应者的 I-PD-L1 + EV 水平升高。利用阶梯楔形微流控芯片可以同时检测PD-L1 + EV亚型,有助于精确预测口腔癌免疫治疗结果。































 京公网安备 11010802027423号
京公网安备 11010802027423号