当前位置:
X-MOL 学术
›
J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Asymmetric Remote Aldol Cyclization Reaction to Synthesize Trifluoromethylated Heterospirocyclic Frameworks
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2024-09-05 , DOI: 10.1021/acs.joc.4c01839 José Trujillo-Sierra 1 , José Miguel Sansano 1 , Jorge Pardos 2 , Tomás Tejero 3 , Pedro Merino 2 , María de Gracia Retamosa 1
The Journal of Organic Chemistry ( IF 3.3 ) Pub Date : 2024-09-05 , DOI: 10.1021/acs.joc.4c01839 José Trujillo-Sierra 1 , José Miguel Sansano 1 , Jorge Pardos 2 , Tomás Tejero 3 , Pedro Merino 2 , María de Gracia Retamosa 1
Affiliation
|
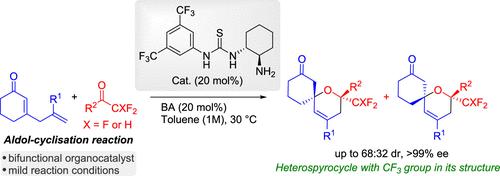
The highly enantioselective organocatalytic synthesis of dihydropyran spirocyclic compounds bearing di- and trifluoromethyl groups by aldol cyclization reaction via trienamine using cyclic 2,5-dienones and different di- and trifluoromethylketones is described. Using a bifunctional aminothiourea catalyst, trifluoromethyl-functionalized dihydropyran spirocyclic products were obtained with good yields and enantioselectivities. Subsequent transformation with H2 and Pd/C has allowed the synthesis of the tetrahydropyran structure with three stereocenters. The plausible reaction mechanism was investigated by computational methods.
中文翻译:

不对称远程羟醛环化反应合成三氟甲基化杂螺环骨架
描述了使用环状 2,5-二酮和不同的二氟甲基酮和三氟甲基酮,通过三烯胺进行醇醛环化反应,高度对映选择性地有机催化合成带有二氟甲基和三氟甲基的二氢吡喃螺环化合物。使用双功能氨基硫脲催化剂,获得了三氟甲基功能化的二氢吡喃螺环产物,具有良好的收率和对映选择性。随后用H 2和Pd/C进行转化使得能够合成具有三个立构中心的四氢吡喃结构。通过计算方法研究了合理的反应机理。
更新日期:2024-09-05
中文翻译:

不对称远程羟醛环化反应合成三氟甲基化杂螺环骨架
描述了使用环状 2,5-二酮和不同的二氟甲基酮和三氟甲基酮,通过三烯胺进行醇醛环化反应,高度对映选择性地有机催化合成带有二氟甲基和三氟甲基的二氢吡喃螺环化合物。使用双功能氨基硫脲催化剂,获得了三氟甲基功能化的二氢吡喃螺环产物,具有良好的收率和对映选择性。随后用H 2和Pd/C进行转化使得能够合成具有三个立构中心的四氢吡喃结构。通过计算方法研究了合理的反应机理。































 京公网安备 11010802027423号
京公网安备 11010802027423号