当前位置:
X-MOL 学术
›
J. Med. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Transferrin-Modified Carprofen Platinum(IV) Nanoparticles as Antimetastasis Agents with Tumor Targeting, Inflammation Inhibition, Epithelial–Mesenchymal Transition Suppression, and Immune Activation Properties
Journal of Medicinal Chemistry ( IF 6.8 ) Pub Date : 2024-09-05 , DOI: 10.1021/acs.jmedchem.4c01265 Ming Zhang 1 , Yan Chen 1, 2 , Shuaiqi Feng 1 , Yanqin He 1 , Zhifang Liu 1 , Ning Zhang 1 , Qingpeng Wang 1
Journal of Medicinal Chemistry ( IF 6.8 ) Pub Date : 2024-09-05 , DOI: 10.1021/acs.jmedchem.4c01265 Ming Zhang 1 , Yan Chen 1, 2 , Shuaiqi Feng 1 , Yanqin He 1 , Zhifang Liu 1 , Ning Zhang 1 , Qingpeng Wang 1
Affiliation
|
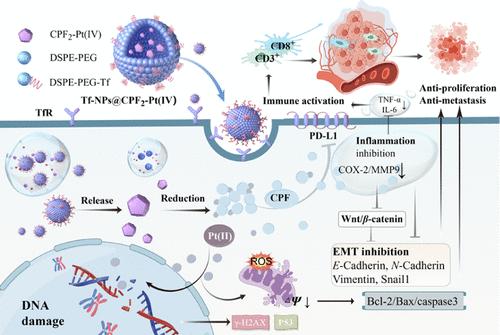
The inflammatory microenvironment is a central driver of tumor metastasis, intimately associated with the promotion of epithelial–mesenchymal transition (EMT) and immune suppression. Here, transferrin-modified carprofen platinum(IV) nanoparticles Tf-NPs@CPF2–Pt(IV) with promising antiproliferative and antimetastatic properties were developed, which activated by inhibiting inflammation, suppressing EMT, and activating immune responses besides causing DNA injury. The nanoparticles released the active ingredient CPF2–Pt(IV) in a sustained manner and offered enhanced pharmacokinetic properties compared to free CPF2–Pt(IV) in vivo. Additionally, they possessed satisfactory tumor targeting effects via the transferrin motif. Serious DNA damage was induced with the upregulation of γ-H2AX and P53, and the mitochondria-mediated apoptotic pathway Bcl-2/Bax/caspase3 was initiated. Inflammation was alleviated by inhibiting COX-2 and MMP9 and decreasing inflammatory cytokines TNF-α and IL-6. Subsequently, the EMT was reversed by inhibiting the Wnt/β-catenin pathway. Furthermore, the antitumor immunity was provoked by blocking the immune checkpoint PD-L1 and increasing CD3+ and CD8+ T lymphocytes in tumors.
中文翻译:

转铁蛋白修饰的卡洛芬铂 (IV) 纳米颗粒作为抗转移剂,具有肿瘤靶向、炎症抑制、上皮-间充质转化抑制和免疫激活特性
炎症微环境是肿瘤转移的核心驱动因素,与促进上皮-间充质转化 (EMT) 和免疫抑制密切相关。在这里,开发了转铁蛋白修饰的卡洛芬铂 (IV) 纳米颗粒 Tf-NPs@CPF2-Pt(IV),具有很好的抗增殖和抗转移特性,除了引起 DNA 损伤外,还通过抑制炎症、抑制 EMT 和激活免疫反应来激活。纳米颗粒持续释放活性成分 CPF2-Pt(IV),与体内游离 CPF2-Pt(IV) 相比,具有增强的药代动力学特性。此外,它们通过转铁蛋白基序具有令人满意的肿瘤靶向作用。γ-H2AX 和 P53 的上调诱导了严重的 DNA 损伤,并启动了线粒体介导的凋亡途径 Bcl-2/Bax/caspase3。通过抑制 COX-2 和 MMP9 以及减少炎性细胞因子 TNF-α 和 IL-6 来缓解炎症。随后,通过抑制 Wnt/β-catenin 通路逆转 EMT。此外,通过阻断免疫检查点 PD-L1 和增加肿瘤中 CD3+ 和 CD8+ T 淋巴细胞来激发抗肿瘤免疫。
更新日期:2024-09-05
中文翻译:

转铁蛋白修饰的卡洛芬铂 (IV) 纳米颗粒作为抗转移剂,具有肿瘤靶向、炎症抑制、上皮-间充质转化抑制和免疫激活特性
炎症微环境是肿瘤转移的核心驱动因素,与促进上皮-间充质转化 (EMT) 和免疫抑制密切相关。在这里,开发了转铁蛋白修饰的卡洛芬铂 (IV) 纳米颗粒 Tf-NPs@CPF2-Pt(IV),具有很好的抗增殖和抗转移特性,除了引起 DNA 损伤外,还通过抑制炎症、抑制 EMT 和激活免疫反应来激活。纳米颗粒持续释放活性成分 CPF2-Pt(IV),与体内游离 CPF2-Pt(IV) 相比,具有增强的药代动力学特性。此外,它们通过转铁蛋白基序具有令人满意的肿瘤靶向作用。γ-H2AX 和 P53 的上调诱导了严重的 DNA 损伤,并启动了线粒体介导的凋亡途径 Bcl-2/Bax/caspase3。通过抑制 COX-2 和 MMP9 以及减少炎性细胞因子 TNF-α 和 IL-6 来缓解炎症。随后,通过抑制 Wnt/β-catenin 通路逆转 EMT。此外,通过阻断免疫检查点 PD-L1 和增加肿瘤中 CD3+ 和 CD8+ T 淋巴细胞来激发抗肿瘤免疫。































 京公网安备 11010802027423号
京公网安备 11010802027423号