当前位置:
X-MOL 学术
›
J. Med. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Structure Activity of β-Amidomethyl Vinyl Sulfones as Covalent Inhibitors of Chikungunya nsP2 Cysteine Protease with Antialphavirus Activity
Journal of Medicinal Chemistry ( IF 6.8 ) Pub Date : 2024-09-05 , DOI: 10.1021/acs.jmedchem.4c01346 Anirban Ghoshal 1, 2 , Kesatebrhan Haile Asressu 1, 2 , Mohammad Anwar Hossain 1, 2 , Peter J Brown 1, 2 , Meganathan Nandakumar 1 , Anand Vala 3 , Eric M Merten 2, 4 , John D Sears 2, 5 , Isabella Law 6 , Jane E Burdick 6 , Noah L Morales 6 , Sumera Perveen 7 , Kenneth H Pearce 2, 4 , Konstantin I Popov 2, 4 , Nathaniel J Moorman 2, 5 , Mark T Heise 2, 5, 6 , Timothy M Willson 1, 2
Journal of Medicinal Chemistry ( IF 6.8 ) Pub Date : 2024-09-05 , DOI: 10.1021/acs.jmedchem.4c01346 Anirban Ghoshal 1, 2 , Kesatebrhan Haile Asressu 1, 2 , Mohammad Anwar Hossain 1, 2 , Peter J Brown 1, 2 , Meganathan Nandakumar 1 , Anand Vala 3 , Eric M Merten 2, 4 , John D Sears 2, 5 , Isabella Law 6 , Jane E Burdick 6 , Noah L Morales 6 , Sumera Perveen 7 , Kenneth H Pearce 2, 4 , Konstantin I Popov 2, 4 , Nathaniel J Moorman 2, 5 , Mark T Heise 2, 5, 6 , Timothy M Willson 1, 2
Affiliation
|
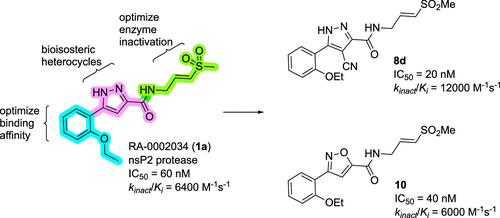
Despite their widespread impact on human health, there are no approved drugs for combating alphavirus infections. The heterocyclic β-aminomethyl vinyl sulfone RA-0002034 (1a) is a potent irreversible covalent inhibitor of the alphavirus nsP2 cysteine protease with broad-spectrum antiviral activity. Analogs of 1a that varied each of the three regions of the molecule were synthesized to establish structure–activity relationships for the inhibition of Chikungunya (CHIKV) nsP2 protease and viral replication. The vinyl sulfone covalent warhead was highly sensitive to modifications. However, alterations to the core five-membered heterocycle and aryl substituent were well tolerated. The 5-(2,5-dimethoxyphenyl)pyrazole (1o) and 4-cyanopyrazole (8d) analogs exhibited kinact/Ki ratios >9000 M–1 s–1. 3-Arylisoxazole (10) was identified as an isosteric replacement for the five-membered heterocycle, which circumvented the intramolecular cyclization of pyrazole-based inhibitors like 1a. A ligand-based model of the enzyme active site was developed to aid the design of nsP2 protease inhibitors as potential therapeutics against alphaviruses.
中文翻译:

β-酰胺甲基乙烯基砜作为具有抗α病毒活性的基孔肯雅热nsP2半胱氨酸蛋白酶共价抑制剂的结构活性
尽管它们对人类健康有广泛的影响,但目前还没有获批的药物来对抗甲病毒感染。杂环 β-氨基甲基乙烯基砜 RA-0002034 (1a) 是一种有效的、不可逆的甲病毒 nsP2 半胱氨酸蛋白酶共价抑制剂,具有广谱抗病毒活性。合成了改变分子三个区域中每个区域的 1a 类似物,以建立抑制基孔肯雅热 (CHIKV) nsP2 蛋白酶和病毒复制的结构-活性关系。乙烯基砜共价弹头对修饰高度敏感。然而,核心五元杂环和芳基取代基的改变具有良好的耐受性。5-(2,5-二甲氧基苯基)吡唑 (1o) 和 4-氰基吡唑 (8d) 类似物表现出 k无效/K 比率 >9000 M–1 s–1。3-芳基恶唑 (10) 被鉴定为五元杂环的等构替代物,它规避了吡唑类抑制剂(如 1a)的分子内环化。开发了一种基于配体的酶活性位点模型,以帮助设计 nsP2 蛋白酶抑制剂作为针对甲病毒的潜在疗法。
更新日期:2024-09-05
中文翻译:

β-酰胺甲基乙烯基砜作为具有抗α病毒活性的基孔肯雅热nsP2半胱氨酸蛋白酶共价抑制剂的结构活性
尽管它们对人类健康有广泛的影响,但目前还没有获批的药物来对抗甲病毒感染。杂环 β-氨基甲基乙烯基砜 RA-0002034 (1a) 是一种有效的、不可逆的甲病毒 nsP2 半胱氨酸蛋白酶共价抑制剂,具有广谱抗病毒活性。合成了改变分子三个区域中每个区域的 1a 类似物,以建立抑制基孔肯雅热 (CHIKV) nsP2 蛋白酶和病毒复制的结构-活性关系。乙烯基砜共价弹头对修饰高度敏感。然而,核心五元杂环和芳基取代基的改变具有良好的耐受性。5-(2,5-二甲氧基苯基)吡唑 (1o) 和 4-氰基吡唑 (8d) 类似物表现出 k无效/K 比率 >9000 M–1 s–1。3-芳基恶唑 (10) 被鉴定为五元杂环的等构替代物,它规避了吡唑类抑制剂(如 1a)的分子内环化。开发了一种基于配体的酶活性位点模型,以帮助设计 nsP2 蛋白酶抑制剂作为针对甲病毒的潜在疗法。































 京公网安备 11010802027423号
京公网安备 11010802027423号