当前位置:
X-MOL 学术
›
J. Mater. Chem. A
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Functional group tuning of CAU-10(Al) for efficient C2H2 storage and C2H2/CO2 separation
Journal of Materials Chemistry A ( IF 10.7 ) Pub Date : 2024-09-04 , DOI: 10.1039/d4ta02821j Eun Woo Lee , Balkaran Singh Sran , Ayoub Daouli , Maftun Salimov , Ji Woong Yoon , Kyung Ho Cho , Donghui Jo , Guillaume Maurin , Sukyung Lee , U-Hwang Lee
Journal of Materials Chemistry A ( IF 10.7 ) Pub Date : 2024-09-04 , DOI: 10.1039/d4ta02821j Eun Woo Lee , Balkaran Singh Sran , Ayoub Daouli , Maftun Salimov , Ji Woong Yoon , Kyung Ho Cho , Donghui Jo , Guillaume Maurin , Sukyung Lee , U-Hwang Lee
|
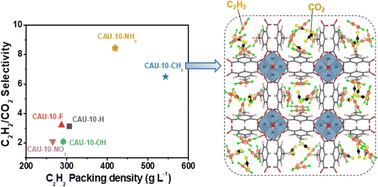
Adsorptive separation of acetylene (C2H2) from a C2H2/CO2 mixture has emerged as an alternative to replace cryogenic distillation to obtain high purity C2H2 in a safer manner. Al-based metal–organic frameworks, such as MIL-160 and CAU-10–NH2, have been reported as good acetylene adsorbents with high selectivity and high acetylene packing density. According to this, incorporation of hetero atoms in the MOF pore wall clearly has a positive impact on enhancing C2H2 capture performance. Nonetheless, a systematic evaluation of the role played by functional groups within the same MOF structure in their C2H2 sorption performance is still required. In this study, CAU-10 samples were prepared according to the functional group using Al metal ions and various functionalized organic linkers, including pristine terephthalate (–H) and its modified forms (–OH, –F, –NO2, –NH2, and –CH3) to investigate their C2H2 and CO2 adsorption properties. The C2H2/CO2 separation performance of the adsorbents was investigated using single gas adsorption isotherms and by application of the ideal adsorbed solution theory as well as dynamic breakthrough experiments for the gas mixture under dry and humid conditions. Interestingly, CAU-10–CH3 was demonstrated to exhibit a record high packing density of 543 g L−1 at 25 °C for acetylene, 2.8 times higher than the CO2 uptake at 1 bar. A breakthrough mixture experiment using CAU-10–CH3, and CAU-10–NH2 as comparative samples confirmed CAU-10–CH3 as a prominent candidate for acetylene selective adsorption under both dry and humid conditions. The microscopic origin of these attractive adsorption/separation performances was understood from density functional theory calculations and single component/binary mixture grand canonical Monte Carlo simulations.
中文翻译:

CAU-10(Al) 的官能团调整可实现高效 C2H2 存储和 C2H2/CO2 分离
从C 2 H 2 /CO 2混合物中吸附分离乙炔(C 2 H 2 )已成为替代低温蒸馏的替代方案,从而以更安全的方式获得高纯度C 2 H 2 。铝基金属有机骨架,如MIL-160和CAU-10-NH 2 ,已被报道为良好的乙炔吸附剂,具有高选择性和高乙炔填充密度。据此,MOF孔壁中杂原子的掺入显然对增强C 2 H 2捕获性能具有积极影响。尽管如此,仍然需要对同一MOF结构内的官能团在其C 2 H 2吸附性能中所起的作用进行系统评估。在本研究中,CAU-10样品根据官能团使用Al金属离子和各种官能化有机连接体制备,包括原始对苯二甲酸酯(–H)及其改性形式(–OH、–F、–NO 2 、–NH 2 ,和-CH 3 )来研究它们的C 2 H 2和CO 2吸附性能。 利用单一气体吸附等温线,应用理想吸附溶液理论以及干湿条件下气体混合物的动态突破实验,研究了吸附剂的C 2 H 2 /CO 2分离性能。有趣的是,CAU-10–CH 3被证明在 25 °C 下乙炔表现出创纪录的 543 g L -1堆积密度,比 1 bar 下的 CO 2吸收高 2.8 倍。使用 CAU-10–CH 3和 CAU-10–NH 2作为比较样品进行的突破性混合物实验证实,CAU-10–CH 3是干燥和潮湿条件下乙炔选择性吸附的重要候选者。这些有吸引力的吸附/分离性能的微观起源可以通过密度泛函理论计算和单组分/二元混合物大正则蒙特卡罗模拟来理解。
更新日期:2024-09-04
中文翻译:

CAU-10(Al) 的官能团调整可实现高效 C2H2 存储和 C2H2/CO2 分离
从C 2 H 2 /CO 2混合物中吸附分离乙炔(C 2 H 2 )已成为替代低温蒸馏的替代方案,从而以更安全的方式获得高纯度C 2 H 2 。铝基金属有机骨架,如MIL-160和CAU-10-NH 2 ,已被报道为良好的乙炔吸附剂,具有高选择性和高乙炔填充密度。据此,MOF孔壁中杂原子的掺入显然对增强C 2 H 2捕获性能具有积极影响。尽管如此,仍然需要对同一MOF结构内的官能团在其C 2 H 2吸附性能中所起的作用进行系统评估。在本研究中,CAU-10样品根据官能团使用Al金属离子和各种官能化有机连接体制备,包括原始对苯二甲酸酯(–H)及其改性形式(–OH、–F、–NO 2 、–NH 2 ,和-CH 3 )来研究它们的C 2 H 2和CO 2吸附性能。 利用单一气体吸附等温线,应用理想吸附溶液理论以及干湿条件下气体混合物的动态突破实验,研究了吸附剂的C 2 H 2 /CO 2分离性能。有趣的是,CAU-10–CH 3被证明在 25 °C 下乙炔表现出创纪录的 543 g L -1堆积密度,比 1 bar 下的 CO 2吸收高 2.8 倍。使用 CAU-10–CH 3和 CAU-10–NH 2作为比较样品进行的突破性混合物实验证实,CAU-10–CH 3是干燥和潮湿条件下乙炔选择性吸附的重要候选者。这些有吸引力的吸附/分离性能的微观起源可以通过密度泛函理论计算和单组分/二元混合物大正则蒙特卡罗模拟来理解。































 京公网安备 11010802027423号
京公网安备 11010802027423号