当前位置:
X-MOL 学术
›
Phys. Chem. Chem. Phys.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Rational design of anthocyanidins-directed near-infrared two-photon fluorescent probes
Physical Chemistry Chemical Physics ( IF 2.9 ) Pub Date : 2024-09-04 , DOI: 10.1039/d4cp02067g Xiu-E Zhang 1 , Xue Wei 2 , Wei-Bo Cui 2 , Jin-Pu Bai 1 , Aynur Matyusup 1 , Jing-Fu Guo 1 , Hui Li 2 , Ai-Min Ren 2
Physical Chemistry Chemical Physics ( IF 2.9 ) Pub Date : 2024-09-04 , DOI: 10.1039/d4cp02067g Xiu-E Zhang 1 , Xue Wei 2 , Wei-Bo Cui 2 , Jin-Pu Bai 1 , Aynur Matyusup 1 , Jing-Fu Guo 1 , Hui Li 2 , Ai-Min Ren 2
Affiliation
|
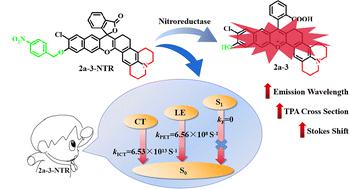
Recently, two-photon fluorescent probes based on anthocyanidin molecules have attracted extensive attention due to their outstanding photophysical properties. However, there are only a few two-photon excited fluorescent probes that really meet the requirements of relatively long emission wavelengths (>600 nm), large two-photon absorption (TPA) cross-sections (300 GM), significant Stokes shift (>80 nm), and high fluorescence intensity. Herein, the photophysical properties of a series of anthocyanidins with the same substituents but different fluorophore skeletons are investigated in detail. Compared with b-series molecules, a-series molecules with a six-membered ring in the backbone have a slightly higher reorganization energy. This results in more energy loss upon light excitation, enabling the reaction products to detect NTR through a larger Stokes shift. More importantly, there is very little decrease in fluorescence intensity as the Stokes shift increases. These features are extremely valuable for high-resolution NTR detection. In light of this, novel 2a-n (n = 1–5) compounds are designed, which are accomplished by inhibiting the twisted intramolecular charge transfer (TICT) effect through alkyl cyclization, azetidine ring and extending π conjugation. Among them, 2a-3 gains a long emission spectrum (λem = 691.4 nm), noticeable TPA cross-section (957 GM), and large Stokes shift (110 nm), indicating that it serves as a promising candidate for two-photon fluorescent dyes. It is hoped that this work will offer some insightful theoretical direction for the development of novel high performance anthocyanin fluorescent materials.
中文翻译:

花青素定向近红外双光子荧光探针的合理设计
近年来,基于花青素分子的双光子荧光探针因其优异的光物理性质而受到广泛关注。然而,真正满足较长发射波长(>600 nm)、大双光子吸收(TPA)截面(300 GM)、显着斯托克斯位移(% 3E80 nm),荧光强度高。在此,详细研究了一系列具有相同取代基但不同荧光团骨架的花青素的光物理性质。与b系列分子相比,主链具有六元环的a系列分子具有稍高的重组能。这导致光激发时更多的能量损失,使反应产物能够通过更大的斯托克斯位移来检测 NTR。更重要的是,随着斯托克斯位移的增加,荧光强度几乎没有降低。这些功能对于高分辨率 NTR 检测非常有价值。鉴于此,设计了新型2a- n ( n =1-5)化合物,该化合物是通过烷基环化、氮杂环丁烷环和延长π共轭来抑制扭曲分子内电荷转移(TICT)效应来实现的。其中, 2a-3具有长发射光谱( λ em = 691.4 nm)、明显的TPA截面(957 GM)和大斯托克斯位移(110 nm),表明它是双光子的有希望的候选者荧光染料。 希望这项工作能够为新型高性能花青素荧光材料的开发提供一些富有洞察力的理论方向。
更新日期:2024-09-05
中文翻译:

花青素定向近红外双光子荧光探针的合理设计
近年来,基于花青素分子的双光子荧光探针因其优异的光物理性质而受到广泛关注。然而,真正满足较长发射波长(>600 nm)、大双光子吸收(TPA)截面(300 GM)、显着斯托克斯位移(% 3E80 nm),荧光强度高。在此,详细研究了一系列具有相同取代基但不同荧光团骨架的花青素的光物理性质。与b系列分子相比,主链具有六元环的a系列分子具有稍高的重组能。这导致光激发时更多的能量损失,使反应产物能够通过更大的斯托克斯位移来检测 NTR。更重要的是,随着斯托克斯位移的增加,荧光强度几乎没有降低。这些功能对于高分辨率 NTR 检测非常有价值。鉴于此,设计了新型2a- n ( n =1-5)化合物,该化合物是通过烷基环化、氮杂环丁烷环和延长π共轭来抑制扭曲分子内电荷转移(TICT)效应来实现的。其中, 2a-3具有长发射光谱( λ em = 691.4 nm)、明显的TPA截面(957 GM)和大斯托克斯位移(110 nm),表明它是双光子的有希望的候选者荧光染料。 希望这项工作能够为新型高性能花青素荧光材料的开发提供一些富有洞察力的理论方向。































 京公网安备 11010802027423号
京公网安备 11010802027423号