当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Chemoselective N-Heterocyclic Carbene-Catalyzed Cross-Benzoin Reactions: Importance of the Fused Ring in Triazolium Salts
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2014-05-16 , DOI: 10.1021/ja501772m Steven M. Langdon 1 , Myron M. D. Wilde 1 , Karen Thai 1 , Michel Gravel 1
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2014-05-16 , DOI: 10.1021/ja501772m Steven M. Langdon 1 , Myron M. D. Wilde 1 , Karen Thai 1 , Michel Gravel 1
Affiliation
|
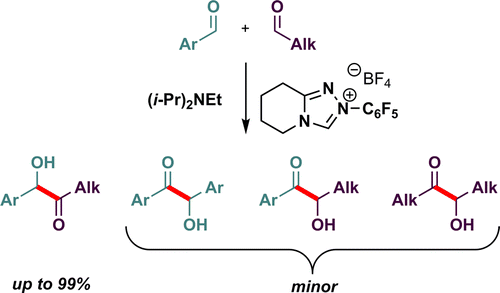
Morpholinone- and piperidinone-derived triazolium salts are shown to catalyze highly chemoselective cross-benzoin reactions between aliphatic and aromatic aldehydes. The reaction scope includes ortho-, meta-, and para-substituted benzaldehyde derivatives with a range of electron-donating and -withdrawing groups as well as branched and unbranched aliphatic aldehydes. Catalytic loadings as low as 5 mol % give excellent yields in these reactions (up to 99%).
中文翻译:

化学选择性 N-杂环卡宾催化的交叉安息香反应:三唑鎓盐中稠环的重要性
吗啉酮和哌啶酮衍生的三唑鎓盐可催化脂肪族醛和芳香醛之间的高度化学选择性交叉安息香反应。反应范围包括具有一系列给电子和吸电子基团的邻位、间位和对位取代的苯甲醛衍生物以及支链和未支链的脂肪族醛。低至 5 mol% 的催化负载量在这些反应中提供了极好的产率(高达 99%)。
更新日期:2014-05-16
中文翻译:

化学选择性 N-杂环卡宾催化的交叉安息香反应:三唑鎓盐中稠环的重要性
吗啉酮和哌啶酮衍生的三唑鎓盐可催化脂肪族醛和芳香醛之间的高度化学选择性交叉安息香反应。反应范围包括具有一系列给电子和吸电子基团的邻位、间位和对位取代的苯甲醛衍生物以及支链和未支链的脂肪族醛。低至 5 mol% 的催化负载量在这些反应中提供了极好的产率(高达 99%)。





















































 京公网安备 11010802027423号
京公网安备 11010802027423号