当前位置:
X-MOL 学术
›
ACS Energy Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
The Potential of Zero Total Charge Predicts Cation Effects for the Oxygen Reduction Reaction
ACS Energy Letters ( IF 19.3 ) Pub Date : 2024-09-04 , DOI: 10.1021/acsenergylett.4c01897 Jay T. Bender 1 , Rohan Yuri Sanspeur 2 , Angel E. Valles 1 , Alyssa K. Uvodich 1 , Delia J. Milliron 1, 3 , John R. Kitchin 2 , Joaquin Resasco 1
ACS Energy Letters ( IF 19.3 ) Pub Date : 2024-09-04 , DOI: 10.1021/acsenergylett.4c01897 Jay T. Bender 1 , Rohan Yuri Sanspeur 2 , Angel E. Valles 1 , Alyssa K. Uvodich 1 , Delia J. Milliron 1, 3 , John R. Kitchin 2 , Joaquin Resasco 1
Affiliation
|
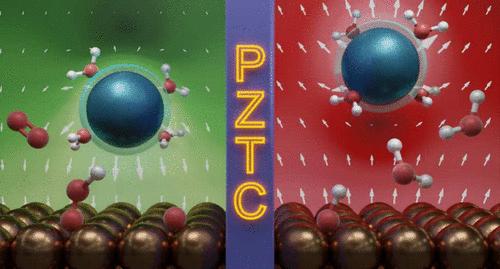
Cation effects are frequently observed for electrochemical reactions that take place at strongly reducing potentials. But a clear understanding of when cation effects will be observed for chemistries like the oxygen reduction reaction (ORR), which occurs at more mildly reducing potentials, has not been developed. Here, based on the results of experimental and computational studies, we propose that the potential of zero total charge (PZTC) predicts whether ORR rates will be influenced by alkali metal cation size. For metals whose PZTC is positive of the ORR potential window (Pt, Ir, Ru, and Au), the surface is negatively charged during catalysis, allowing cations to accumulate in the double layer and influence the stability of reaction intermediates. For these metals, ORR rates increase with cation size (Li+ < Na+ < K+ < Cs+). We argue that interfacial cations decrease *OH poisoning over strongly binding catalysts whose rates are limited by product desorption and decrease the apparent activation barrier for O2 adsorption over weakly binding catalysts. Conversely, for metals whose PZTC is negative of the ORR potential window (Ag and Pd), the surface is positively charged; therefore, cations are electrostatically repelled from the surface under reaction conditions. Their corresponding ORR rates are insensitive to the electrolyte composition.
中文翻译:

零总电荷的电势预测了氧还原反应的阳离子效应
在强还原电位下发生的电化学反应中经常观察到阳离子效应。但对于氧还原反应 (ORR) 等化学反应何时观察到阳离子效应(ORR 发生在较温和的还原电位下)尚未形成清晰的认识。在这里,根据实验和计算研究的结果,我们提出零总电荷电势 (PZTC) 可以预测 ORR 速率是否会受到碱金属阳离子尺寸的影响。对于PZTC为ORR电位窗口正值的金属(Pt、Ir、Ru和Au),催化过程中表面带负电,使阳离子在双层中积累,影响反应中间体的稳定性。对于这些金属,ORR 率随着阳离子尺寸的增加而增加 (Li + < Na + < K + < Cs + )。我们认为,界面阳离子可以减少强结合催化剂上的 *OH 中毒,其速率受到产物解吸的限制,并降低弱结合催化剂上 O 2吸附的表观活化势垒。相反,对于 PZTC 为 ORR 电势窗口负值的金属(Ag 和 Pd),表面带正电;因此,在反应条件下,阳离子会被表面静电排斥。它们相应的 ORR 率对电解质成分不敏感。
更新日期:2024-09-04
中文翻译:

零总电荷的电势预测了氧还原反应的阳离子效应
在强还原电位下发生的电化学反应中经常观察到阳离子效应。但对于氧还原反应 (ORR) 等化学反应何时观察到阳离子效应(ORR 发生在较温和的还原电位下)尚未形成清晰的认识。在这里,根据实验和计算研究的结果,我们提出零总电荷电势 (PZTC) 可以预测 ORR 速率是否会受到碱金属阳离子尺寸的影响。对于PZTC为ORR电位窗口正值的金属(Pt、Ir、Ru和Au),催化过程中表面带负电,使阳离子在双层中积累,影响反应中间体的稳定性。对于这些金属,ORR 率随着阳离子尺寸的增加而增加 (Li + < Na + < K + < Cs + )。我们认为,界面阳离子可以减少强结合催化剂上的 *OH 中毒,其速率受到产物解吸的限制,并降低弱结合催化剂上 O 2吸附的表观活化势垒。相反,对于 PZTC 为 ORR 电势窗口负值的金属(Ag 和 Pd),表面带正电;因此,在反应条件下,阳离子会被表面静电排斥。它们相应的 ORR 率对电解质成分不敏感。

































 京公网安备 11010802027423号
京公网安备 11010802027423号