当前位置:
X-MOL 学术
›
ACS Energy Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Spontaneous Oxidation of Organic Matter and Ammonium Uptake from Manure Wastewater by Redox-Active Materials
ACS Energy Letters ( IF 19.3 ) Pub Date : 2024-09-03 , DOI: 10.1021/acsenergylett.4c01896 Rui Wang 1 , Anneke Moeller 1 , Hanyu Tang 2 , Paulo Falco Cobra 3 , Mohan Qin 2 , Song Jin 1
ACS Energy Letters ( IF 19.3 ) Pub Date : 2024-09-03 , DOI: 10.1021/acsenergylett.4c01896 Rui Wang 1 , Anneke Moeller 1 , Hanyu Tang 2 , Paulo Falco Cobra 3 , Mohan Qin 2 , Song Jin 1
Affiliation
|
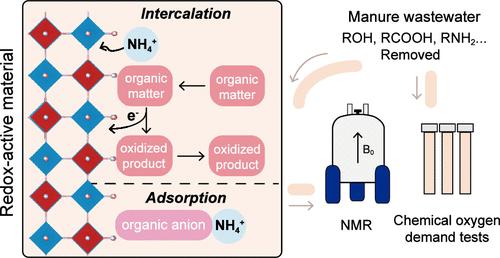
Spontaneous NH4+ uptake using solid-state redox-active materials, driven by the oxidation of organic matter in manure wastewater, provides a sustainable and energy-friendly method for nutrient recovery. However, the mechanisms of electron transfer and accompanying ion transport are poorly understood. Here, we investigated the electron transfer pathway and NH4+ uptake mechanism by analyzing the composition changes in manure wastewater via NMR and the corresponding electrochemical results. We found that the spontaneous NH4+ uptake involves a direct electron transfer process without redox mediators, with the co-occurrence of ion intercalation and adsorption. Based on the elucidated mechanisms, a core–shell Prussian blue analogue redox material with improved stability in manure wastewater and enhanced NH4+ recovery compared to previously reported electrode materials was developed. Such a fundamental understanding of the oxidation of organic compounds and spontaneous NH4+ uptake by redox-active materials can guide the design of redox materials for effective resource recovery and environmental applications.
中文翻译:

氧化还原活性材料对有机物的自发氧化和粪便废水中氨的吸收
在粪便废水中有机物氧化的驱动下,使用固态氧化还原活性材料自发吸收NH 4 + ,为养分回收提供了一种可持续且能源友好的方法。然而,人们对电子转移和伴随的离子传输的机制知之甚少。在这里,我们通过核磁共振分析粪便废水的成分变化和相应的电化学结果,研究了电子转移途径和NH 4 +吸收机制。我们发现自发的NH 4 +吸收涉及一个没有氧化还原介体的直接电子转移过程,同时发生离子嵌入和吸附。基于阐明的机制,开发了一种核壳普鲁士蓝类似氧化还原材料,与之前报道的电极材料相比,该材料在粪便废水中具有更高的稳定性,并且具有更高的NH 4 +回收率。对有机化合物的氧化和氧化还原活性材料自发吸收NH 4 +的这种基本了解可以指导氧化还原材料的设计,以实现有效的资源回收和环境应用。
更新日期:2024-09-03
中文翻译:

氧化还原活性材料对有机物的自发氧化和粪便废水中氨的吸收
在粪便废水中有机物氧化的驱动下,使用固态氧化还原活性材料自发吸收NH 4 + ,为养分回收提供了一种可持续且能源友好的方法。然而,人们对电子转移和伴随的离子传输的机制知之甚少。在这里,我们通过核磁共振分析粪便废水的成分变化和相应的电化学结果,研究了电子转移途径和NH 4 +吸收机制。我们发现自发的NH 4 +吸收涉及一个没有氧化还原介体的直接电子转移过程,同时发生离子嵌入和吸附。基于阐明的机制,开发了一种核壳普鲁士蓝类似氧化还原材料,与之前报道的电极材料相比,该材料在粪便废水中具有更高的稳定性,并且具有更高的NH 4 +回收率。对有机化合物的氧化和氧化还原活性材料自发吸收NH 4 +的这种基本了解可以指导氧化还原材料的设计,以实现有效的资源回收和环境应用。































 京公网安备 11010802027423号
京公网安备 11010802027423号