当前位置:
X-MOL 学术
›
J. Med. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Re-Evaluating PIN1 as a Therapeutic Target in Oncology Using Neutral Inhibitors and PROTACs
Journal of Medicinal Chemistry ( IF 6.8 ) Pub Date : 2024-09-04 , DOI: 10.1021/acs.jmedchem.4c01412 Chuan Liu 1 , Zhonghui Chen 2 , Tao Chen 1 , Hongmei Song 2 , Jianbo Shen 1 , Xiaoxi Yuan 2 , Shuai Xia 1 , Qian Liu 2 , Qiuxia Chen 1 , Qiang Tian 2 , Xiaoyun Meng 1 , Zhu Han 2 , Xiaofei Dong 1 , Yu Yang 2 , Longying Cai 1 , Xuemin Cheng 1 , Yangyang Jia 2 , Guansai Liu 1 , Jin Li 1 , Junyou Ge 2 , Dengfeng Dou 1
Journal of Medicinal Chemistry ( IF 6.8 ) Pub Date : 2024-09-04 , DOI: 10.1021/acs.jmedchem.4c01412 Chuan Liu 1 , Zhonghui Chen 2 , Tao Chen 1 , Hongmei Song 2 , Jianbo Shen 1 , Xiaoxi Yuan 2 , Shuai Xia 1 , Qian Liu 2 , Qiuxia Chen 1 , Qiang Tian 2 , Xiaoyun Meng 1 , Zhu Han 2 , Xiaofei Dong 1 , Yu Yang 2 , Longying Cai 1 , Xuemin Cheng 1 , Yangyang Jia 2 , Guansai Liu 1 , Jin Li 1 , Junyou Ge 2 , Dengfeng Dou 1
Affiliation
|
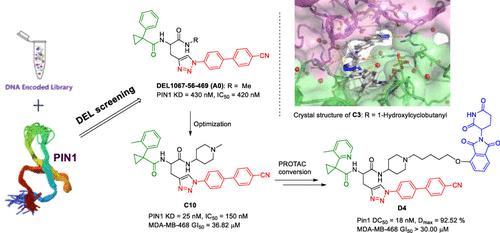
Peptidyl-prolyl cis–trans isomerase NIMA-interacting 1 (PIN1) has emerged as a promising therapeutic target for cancer treatment. However, the current PIN1 inhibitors have shown limited efficacy in animal models, leaving the question of whether PIN1 is a proper oncologic target still unanswered. By screening a 1 trillion DNA-encoded library (DEL), we identified novel nonacidic compounds. Among resynthesized DEL compounds, DEL1067-56-469 (A0) is the most potent one (KD = 430 nM, IC50 = 420 nM). Further optimization of A0 resulted in compound C10 with much improved potency (KD = 25 nM, IC50 = 150 nM). As an alternative approach, C10 was then converted into proteolysis targeting chimeras (PROTACs) in order to achieve deeper downregulation of the PIN1 protein in cancer cell lines. Unfortunately, neither PIN1 inhibitors nor PIN1 PROTACs demonstrated meaningful antiproliferation activity. In addition, siRNA knock-down experiments provided unfavorable evidence of PIN1 as an oncologic target. Our findings highlight the complexity of targeting PIN1 for cancer therapy.
中文翻译:

使用中性抑制剂和 PROTAC 重新评估 PIN1 作为肿瘤学治疗靶点
肽基脯氨酰顺反异构酶 NIMA 相互作用 1 (PIN1) 已成为癌症治疗的一个有前途的治疗靶点。然而,目前的 PIN1 抑制剂在动物模型中显示出的功效有限,因此 PIN1 是否是适当的肿瘤学靶点的问题仍未得到解答。通过筛选 1 万亿 DNA 编码库 (DEL),我们鉴定出了新型非酸性化合物。在重新合成的 DEL 化合物中, DEL1067 - 56 - 469 ( A0 ) 是最有效的一种 (KD = 430 nM,IC 50 = 420 nM)。 A0的进一步优化导致化合物C10 的效力大大提高(KD = 25 nM,IC 50 = 150 nM)。作为一种替代方法, C10随后被转化为蛋白水解靶向嵌合体 (PROTAC),以实现癌细胞系中 PIN1 蛋白的更深层次下调。不幸的是,PIN1 抑制剂和 PIN1 PROTAC 均未表现出有意义的抗增殖活性。此外,siRNA 敲除实验提供了 PIN1 作为肿瘤学靶标的不利证据。我们的研究结果凸显了针对 PIN1 进行癌症治疗的复杂性。
更新日期:2024-09-04
中文翻译:

使用中性抑制剂和 PROTAC 重新评估 PIN1 作为肿瘤学治疗靶点
肽基脯氨酰顺反异构酶 NIMA 相互作用 1 (PIN1) 已成为癌症治疗的一个有前途的治疗靶点。然而,目前的 PIN1 抑制剂在动物模型中显示出的功效有限,因此 PIN1 是否是适当的肿瘤学靶点的问题仍未得到解答。通过筛选 1 万亿 DNA 编码库 (DEL),我们鉴定出了新型非酸性化合物。在重新合成的 DEL 化合物中, DEL1067 - 56 - 469 ( A0 ) 是最有效的一种 (KD = 430 nM,IC 50 = 420 nM)。 A0的进一步优化导致化合物C10 的效力大大提高(KD = 25 nM,IC 50 = 150 nM)。作为一种替代方法, C10随后被转化为蛋白水解靶向嵌合体 (PROTAC),以实现癌细胞系中 PIN1 蛋白的更深层次下调。不幸的是,PIN1 抑制剂和 PIN1 PROTAC 均未表现出有意义的抗增殖活性。此外,siRNA 敲除实验提供了 PIN1 作为肿瘤学靶标的不利证据。我们的研究结果凸显了针对 PIN1 进行癌症治疗的复杂性。































 京公网安备 11010802027423号
京公网安备 11010802027423号