Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Disorder-mediated interactions target proteins to specific condensates
Molecular Cell ( IF 14.5 ) Pub Date : 2024-09-03 , DOI: 10.1016/j.molcel.2024.08.017 Nancy De La Cruz 1 , Prashant Pradhan 1 , Reshma T Veettil 1 , Brooke A Conti 2 , Mariano Oppikofer 2 , Benjamin R Sabari 1
Molecular Cell ( IF 14.5 ) Pub Date : 2024-09-03 , DOI: 10.1016/j.molcel.2024.08.017 Nancy De La Cruz 1 , Prashant Pradhan 1 , Reshma T Veettil 1 , Brooke A Conti 2 , Mariano Oppikofer 2 , Benjamin R Sabari 1
Affiliation
|
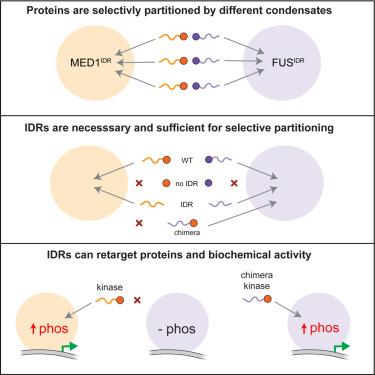
Selective compartmentalization of cellular contents is fundamental to the regulation of biochemistry. Although membrane-bound organelles control composition by using a semi-permeable barrier, biomolecular condensates rely on interactions among constituents to determine composition. Condensates are formed by dynamic multivalent interactions, often involving intrinsically disordered regions (IDRs) of proteins, yet whether distinct compositions can arise from these dynamic interactions is not known. Here, by comparative analysis of proteins differentially partitioned by two different condensates, we find that distinct compositions arise through specific IDR-mediated interactions. The IDRs of differentially partitioned proteins are necessary and sufficient for selective partitioning. Distinct sequence features are required for IDRs to partition, and swapping these sequence features changes the specificity of partitioning. Swapping whole IDRs retargets proteins and their biochemical activity to different condensates. Our results demonstrate that IDR-mediated interactions can target proteins to specific condensates, enabling the spatial regulation of biochemistry within the cell.
中文翻译:

无序介导的相互作用将蛋白质靶向特定的凝聚物
细胞内容的选择性区室化是生物化学调节的基础。尽管膜结合的细胞器通过使用半透性屏障来控制组成,但生物分子凝聚物依赖于成分之间的相互作用来确定组成。缩合物是由动态多价相互作用形成的,通常涉及蛋白质的固有无序区域 (IDR),但尚不清楚这些动态相互作用是否会产生不同的组成。在这里,通过对两种不同凝聚物差异分配的蛋白质进行比较分析,我们发现不同的组成是通过特定的 IDR 介导的相互作用产生的。差异分配蛋白的 IDR 对于选择性分配是必要且足够的。IDR 需要不同的序列特征才能进行分区,交换这些序列特征会改变分区的特异性。交换整个 IDR 将蛋白质及其生化活性重新定位到不同的缩合物中。我们的结果表明,IDR 介导的相互作用可以将蛋白质靶向特定的凝聚物,从而实现细胞内生物化学的空间调节。
更新日期:2024-09-03
中文翻译:

无序介导的相互作用将蛋白质靶向特定的凝聚物
细胞内容的选择性区室化是生物化学调节的基础。尽管膜结合的细胞器通过使用半透性屏障来控制组成,但生物分子凝聚物依赖于成分之间的相互作用来确定组成。缩合物是由动态多价相互作用形成的,通常涉及蛋白质的固有无序区域 (IDR),但尚不清楚这些动态相互作用是否会产生不同的组成。在这里,通过对两种不同凝聚物差异分配的蛋白质进行比较分析,我们发现不同的组成是通过特定的 IDR 介导的相互作用产生的。差异分配蛋白的 IDR 对于选择性分配是必要且足够的。IDR 需要不同的序列特征才能进行分区,交换这些序列特征会改变分区的特异性。交换整个 IDR 将蛋白质及其生化活性重新定位到不同的缩合物中。我们的结果表明,IDR 介导的相互作用可以将蛋白质靶向特定的凝聚物,从而实现细胞内生物化学的空间调节。

































 京公网安备 11010802027423号
京公网安备 11010802027423号