当前位置:
X-MOL 学术
›
Energy Environ. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Low Pt loading with lattice strain for direct ethylene glycol fuel cells
Energy & Environmental Science ( IF 32.4 ) Pub Date : 2024-09-03 , DOI: 10.1039/d4ee01255k
Hao Lei 1 , Ninggui Ma 2 , Kaikai Li 1 , Yu Wang 1 , Qunhui Yuan 1 , Jun Fan 2 , Jianglan Shui 3 , Yan Huang 1, 4, 5
Energy & Environmental Science ( IF 32.4 ) Pub Date : 2024-09-03 , DOI: 10.1039/d4ee01255k
Hao Lei 1 , Ninggui Ma 2 , Kaikai Li 1 , Yu Wang 1 , Qunhui Yuan 1 , Jun Fan 2 , Jianglan Shui 3 , Yan Huang 1, 4, 5
Affiliation
|
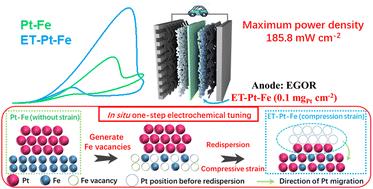
Low Pt loading electrodes are pivotal but challenging in direct ethylene glycol fuel cells (DEGFCs), necessitating a substantial enhancement in both the active site quantity and the catalytic capacity of Pt, which is a long-lasting contradiction in commonly used Pt alloy catalysts. Imparting a certain strain to Pt has been demonstrated to be effective in enhancing Pt activity, which is expected to resolve this bottleneck in alloy catalysts with high Pt weight fractions. However, conventional strain imposition strategies are disadvantageous under harsh conditions of high temperature and pressure as well as high facility requirements. Here, we activated Fe vacancies, induced the rearrangement of Pt atoms, and thereby successfully introduced compressive strain through an in situ facile one-step electrochemical process under ambient conditions. Consequently, the obtained Pt–Fe alloy catalyst (95.8 wt% Pt) achieved unprecedentedly high mass activity and specific activity in ethylene glycol oxidation (22.7 A mgPt−1 and 23.4 mA cmPt−2). Of particular note is that the DEGFC achieves the highest power density and the best stability with a very low Pt loading electrode (Pt loading: 0.1 mg cm−2), surpassing all DEGFCs and many direct methanol fuel cells even with decent noble metal loading electrodes (noble metal loading: >1 mg cm−2) reported. Density-functional theory calculations demonstrate that Fe3 vacancies prefer to adsorb Pt atoms mostly compared to other vacancies and atoms, leading to Pt redistribution and compressive strain. In situ FTIR and online mass spectrometry confirmed that the compression-strained Pt–Fe significantly improved the C–C bond cleavage efficiency and resistance to CO poisoning, revealing the intrinsic mechanism of its excellent performance.
中文翻译:

用于直接乙二醇燃料电池的低 Pt 负载和晶格应变
低铂负载电极在直接乙二醇燃料电池(DEGFC)中至关重要但具有挑战性,需要大幅提高铂的活性位点数量和催化能力,这是常用铂合金催化剂中长期存在的矛盾。已证明赋予 Pt 一定的应变可有效提高 Pt 活性,有望解决高 Pt 重量分数合金催化剂的这一瓶颈。然而,传统的应变施加策略在高温高压的恶劣条件以及高设施要求下是不利的。在这里,我们激活了 Fe 空位,诱导 Pt 原子重排,从而在环境条件下通过原位简便的一步电化学过程成功引入了压缩应变。因此,所获得的Pt-Fe合金催化剂(95.8 wt% Pt)在乙二醇氧化中实现了前所未有的高质量活性和比活性(22.7 A mg Pt -1和23.4 mA cm Pt -2 )。特别值得注意的是,DEGFC 使用非常低的 Pt 负载电极(Pt 负载量:0.1 mg cm -2 )实现了最高的功率密度和最佳稳定性,超越了所有 DEGFC 和许多直接甲醇燃料电池,即使使用了相当好的贵金属负载电极(贵金属负载量:>1 mg cm -2 )报告。 密度泛函理论计算表明,与其他空位和原子相比,Fe 3空位更倾向于吸附Pt原子,从而导致Pt重新分布和压缩应变。原位FTIR 和在线质谱证实,压缩应变 Pt-Fe 显着提高了 C-C 键断裂效率和抗 CO 中毒能力,揭示了其优异性能的内在机制。
更新日期:2024-09-03
中文翻译:

用于直接乙二醇燃料电池的低 Pt 负载和晶格应变
低铂负载电极在直接乙二醇燃料电池(DEGFC)中至关重要但具有挑战性,需要大幅提高铂的活性位点数量和催化能力,这是常用铂合金催化剂中长期存在的矛盾。已证明赋予 Pt 一定的应变可有效提高 Pt 活性,有望解决高 Pt 重量分数合金催化剂的这一瓶颈。然而,传统的应变施加策略在高温高压的恶劣条件以及高设施要求下是不利的。在这里,我们激活了 Fe 空位,诱导 Pt 原子重排,从而在环境条件下通过原位简便的一步电化学过程成功引入了压缩应变。因此,所获得的Pt-Fe合金催化剂(95.8 wt% Pt)在乙二醇氧化中实现了前所未有的高质量活性和比活性(22.7 A mg Pt -1和23.4 mA cm Pt -2 )。特别值得注意的是,DEGFC 使用非常低的 Pt 负载电极(Pt 负载量:0.1 mg cm -2 )实现了最高的功率密度和最佳稳定性,超越了所有 DEGFC 和许多直接甲醇燃料电池,即使使用了相当好的贵金属负载电极(贵金属负载量:>1 mg cm -2 )报告。 密度泛函理论计算表明,与其他空位和原子相比,Fe 3空位更倾向于吸附Pt原子,从而导致Pt重新分布和压缩应变。原位FTIR 和在线质谱证实,压缩应变 Pt-Fe 显着提高了 C-C 键断裂效率和抗 CO 中毒能力,揭示了其优异性能的内在机制。



































 京公网安备 11010802027423号
京公网安备 11010802027423号