当前位置:
X-MOL 学术
›
Chem. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
CCC pincer Ru complex-catalyzed C–H vinylation/6π-E-cyclization of aldimines for constructing 4H-pyrido[1,2-a]pyrimidines
Chemical Science ( IF 7.6 ) Pub Date : 2024-09-03 , DOI: 10.1039/d4sc05067c Heng Cai 1 , Yong-Qiang Tu 1, 2 , Qiang Niu 3 , Wen-Ping Xie 2 , Bin Wang 1 , Ka Lu 1 , Zi-Hao Li 2 , Fu-Min Zhang 1 , Xiao-Ming Zhang 1
Chemical Science ( IF 7.6 ) Pub Date : 2024-09-03 , DOI: 10.1039/d4sc05067c Heng Cai 1 , Yong-Qiang Tu 1, 2 , Qiang Niu 3 , Wen-Ping Xie 2 , Bin Wang 1 , Ka Lu 1 , Zi-Hao Li 2 , Fu-Min Zhang 1 , Xiao-Ming Zhang 1
Affiliation
|
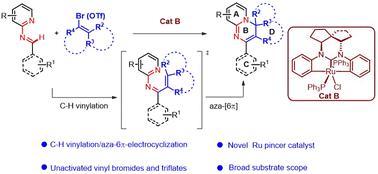
An unusual cascade C–H activation, vinylation and 6π-electrocyclization of 2-pyridyl aldimines with vinyl bromides/triflates was achieved using catalysis with a unique CCC pincer NHC–Ru(III) complex (Cat B). This reaction was found to enable a rapid and diverse synthesis of polycyclic 4H-pyrido[1,2-a]pyrimidine derivatives in mostly good to high yields, and with a broad substrate scope. A mechanistic study suggested the formation of a semi-opened Ru(III) intermediate chelating/activating the aldimine, and the occurrence of single-electron transfer (SET) to generate a vinyl radical, followed by vinylation and then an intramolecular 6π-electrocyclization of 1N,3N-hexatrene to form the product. This protocol provides a convenient approach for preparing and seeking new drug candidates.
中文翻译:

CCC 钳 Ru 配合物催化的醛二胺的 C-H 乙烯基化/6π-E 环化反应,用于构建 4H-吡啶[1,2-a] 嘧啶
使用独特的 CCC 钳形 NHC-Ru(III)配合物 (Cat B) 进行催化,实现了 2-吡啶基二胺与乙烯基溴化物/三氟甲磺酸酯的不寻常级联 C-H 活化、乙烯基化和 6π-电环化。发现该反应能够快速、多样地合成多环 4H-吡啶[1,2-a] 嘧啶衍生物,产量大多为高,底物范围广。一项机理研究表明,形成半开放的 Ru(III) 中间体螯合/激活阿丁胺,并发生单电子转移 (SET) 以产生乙烯基自由基,然后是乙烯基化,然后是 1N,3 N-己三烯的分子内 6π 电环化以形成产物。该方案为准备和寻找新的候选药物提供了一种便捷的方法。
更新日期:2024-09-03
中文翻译:

CCC 钳 Ru 配合物催化的醛二胺的 C-H 乙烯基化/6π-E 环化反应,用于构建 4H-吡啶[1,2-a] 嘧啶
使用独特的 CCC 钳形 NHC-Ru(III)配合物 (Cat B) 进行催化,实现了 2-吡啶基二胺与乙烯基溴化物/三氟甲磺酸酯的不寻常级联 C-H 活化、乙烯基化和 6π-电环化。发现该反应能够快速、多样地合成多环 4H-吡啶[1,2-a] 嘧啶衍生物,产量大多为高,底物范围广。一项机理研究表明,形成半开放的 Ru(III) 中间体螯合/激活阿丁胺,并发生单电子转移 (SET) 以产生乙烯基自由基,然后是乙烯基化,然后是 1N,3 N-己三烯的分子内 6π 电环化以形成产物。该方案为准备和寻找新的候选药物提供了一种便捷的方法。































 京公网安备 11010802027423号
京公网安备 11010802027423号