当前位置:
X-MOL 学术
›
Phys. Chem. Chem. Phys.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
CO2 activation by copper oxide clusters: size, composition, and charge state dependence
Physical Chemistry Chemical Physics ( IF 2.9 ) Pub Date : 2024-09-02 , DOI: 10.1039/d4cp02651a Pavol Mikolaj 1 , Barbara Zamora Yusti 2 , László Nyulászi 2, 3 , Joost M Bakker 4 , Tibor Höltzl 3, 5 , Sandra M Lang 1
Physical Chemistry Chemical Physics ( IF 2.9 ) Pub Date : 2024-09-02 , DOI: 10.1039/d4cp02651a Pavol Mikolaj 1 , Barbara Zamora Yusti 2 , László Nyulászi 2, 3 , Joost M Bakker 4 , Tibor Höltzl 3, 5 , Sandra M Lang 1
Affiliation
|
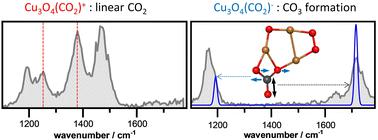
The interaction of CO2 with copper oxide clusters of different size, composition, and charge is investigated via infrared multiple-photon dissociation (IR-MPD) spectroscopy and density functional theory (DFT) calculations. Laser ablation of a copper target in the presence of an O2/He mixture leads to the preferred formation of oxygen-rich copper oxide cluster cations, CuxOy+ (y > x; x ≤ 8), while the anionic cluster distribution is dominated by stoichiometric (x = y) and oxygen-deficient (y < x; x ≤ 8) species. Subsequent reaction of the clusters with CO2 in a flow tube reactor results in the preferred formation of near-stoichiometric CuxOy(CO2)+/− complexes. IR-MPD spectroscopy of the formed complexes reveals the non-activated binding of CO2 to all cations while CO2 is activated by all anions. The great resemblance of spectra for all sizes investigated demonstrates that CO2 activation is largely independent of cluster size and Cu/O ratio but mainly determined by the cluster charge state. Comparison of the IR-MPD spectra with DFT calculations of the model systems Cu2O4(CO2)− and Cu3O4(CO2)− shows that CO2 activation exclusively results in the formation of a CO3 unit. Subsequent CO2 dissociation to CO appears to be unfavorable due to the instability of CO on the copper oxide clusters indicating that potential hydrogenation reactions will most likely proceed via formate or bicarbonate intermediates.
中文翻译:

氧化铜簇活化 CO2:尺寸、成分和电荷状态依赖性
通过红外多光子解离(IR-MPD)光谱和密度泛函理论(DFT)计算研究了CO 2与不同尺寸、组成和电荷的氧化铜簇的相互作用。在 O 2 /He 混合物存在下激光烧蚀铜靶材会优先形成富氧氧化铜簇阳离子 Cu x O y + ( y > x ; x ≤ 8),而阴离子簇分布以化学计量 ( x = y ) 和缺氧 ( y < x ; x ≤ 8) 物种为主。随后在流管反应器中团簇与CO 2的反应导致优选形成接近化学计量的Cu x O y (CO 2 ) +/-络合物。形成的复合物的IR-MPD光谱揭示了CO 2与所有阳离子的非活化结合,而CO 2被所有阴离子活化。所研究的所有尺寸的光谱都非常相似,这表明CO 2活化在很大程度上与团簇尺寸和Cu/O比率无关,但主要由团簇电荷状态决定。 模型系统Cu 2 O 4 (CO 2 ) -和Cu 3 O 4 (CO 2 ) -的IR-MPD光谱与DFT计算的比较表明,CO 2活化仅导致CO 3单元的形成。随后的CO 2解离成CO似乎是不利的,因为氧化铜簇上的CO不稳定,这表明潜在的氢化反应很可能通过甲酸盐或碳酸氢盐中间体进行。
更新日期:2024-09-02
中文翻译:

氧化铜簇活化 CO2:尺寸、成分和电荷状态依赖性
通过红外多光子解离(IR-MPD)光谱和密度泛函理论(DFT)计算研究了CO 2与不同尺寸、组成和电荷的氧化铜簇的相互作用。在 O 2 /He 混合物存在下激光烧蚀铜靶材会优先形成富氧氧化铜簇阳离子 Cu x O y + ( y > x ; x ≤ 8),而阴离子簇分布以化学计量 ( x = y ) 和缺氧 ( y < x ; x ≤ 8) 物种为主。随后在流管反应器中团簇与CO 2的反应导致优选形成接近化学计量的Cu x O y (CO 2 ) +/-络合物。形成的复合物的IR-MPD光谱揭示了CO 2与所有阳离子的非活化结合,而CO 2被所有阴离子活化。所研究的所有尺寸的光谱都非常相似,这表明CO 2活化在很大程度上与团簇尺寸和Cu/O比率无关,但主要由团簇电荷状态决定。 模型系统Cu 2 O 4 (CO 2 ) -和Cu 3 O 4 (CO 2 ) -的IR-MPD光谱与DFT计算的比较表明,CO 2活化仅导致CO 3单元的形成。随后的CO 2解离成CO似乎是不利的,因为氧化铜簇上的CO不稳定,这表明潜在的氢化反应很可能通过甲酸盐或碳酸氢盐中间体进行。































 京公网安备 11010802027423号
京公网安备 11010802027423号