当前位置:
X-MOL 学术
›
Phys. Chem. Chem. Phys.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Photoisomerization mechanism of iminoguanidinium receptors from spectroscopic methods and quantum chemical calculations
Physical Chemistry Chemical Physics ( IF 2.9 ) Pub Date : 2024-09-02 , DOI: 10.1039/d4cp02747g Duy-Khoi Dang 1 , Jeffrey D Einkauf 2 , Xinyou Ma 2 , Radu Custelcean 2 , Ying-Zhong Ma 2 , Paul M Zimmerman 1 , Vyacheslav S Bryantsev 2
Physical Chemistry Chemical Physics ( IF 2.9 ) Pub Date : 2024-09-02 , DOI: 10.1039/d4cp02747g Duy-Khoi Dang 1 , Jeffrey D Einkauf 2 , Xinyou Ma 2 , Radu Custelcean 2 , Ying-Zhong Ma 2 , Paul M Zimmerman 1 , Vyacheslav S Bryantsev 2
Affiliation
|
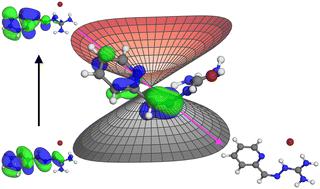
The hydrazone functional group, when coupled with a pyridyl substituent, offers a unique class of widely tunable photoswitches, whose E-to-Z photoisomerization equilibria can be controlled through intramolecular hydrogen bonding between the N–H hydrazone donor and the pyridyl acceptor. However, little is known about the photoisomerization mechanism in this class of compounds. To address this issue, we report a pyridine-appended iminoguanidinium photoswitch that is functionally related to acylhydrazones and provides insight into the photoisomerization processes between the E and Z configurations. The E-to-Z photoisomerization of the E-2-pyridyl-iminoguanidinium cation (2PyMIG) in DMSO, prepared as the bromide salt, was quantified by 1H NMR, and probed in real time with ultrafast laser spectroscopy. The photoisomerization process occurs on a picosecond timescale, resulting in low fluorescence yields. The multiconfigurational reaction path found with the growing string method features a small barrier (4.3 or 6.5 kcal mol−1) that the E isomer in the π–π* state must overcome to reach the minimum energy conical intersection (MECI) connecting the E and Z isomers of 2PyMIG. While two possible pathways exist depending on the orientation of the pyridine ring, both exhibit the same qualitative features along the path and at their MECIs, involving simultaneous changes in the CCNN and CNNC dihedral angles. Furthermore, the ground state barrier for pyridine ring rotation is readily accessible, thus a low barrier pathway to the experimentally observed Z isomer exists for both MECIs leading to a transition from the E isomer to photoproduct. Combining multiconfigurational reaction path calculations using growing string method with time-resolved fluorescence spectroscopy provided crucial insights into the photoisomerization process of 2PyMIG, resulting in both the computational and experimental results pointing to rapid photoisomerization via a surface crossing between the singlet π–π* and the ground states.
中文翻译:

从光谱方法和量子化学计算研究亚氨基胍受体的光异构化机制
腙官能团与吡啶基取代基结合时,提供了一类独特的广泛可调的光开关,其E - Z光异构化平衡可以通过N-H腙供体和吡啶基受体之间的分子内氢键来控制。然而,人们对这类化合物的光致异构化机制知之甚少。为了解决这个问题,我们报道了一种附加吡啶的亚氨基胍光开关,它在功能上与酰腙相关,并提供了对E和Z构型之间光异构化过程的深入了解。 E -2-吡啶基亚氨基胍阳离子 (2PyMIG) 在 DMSO(以溴化物盐形式制备)中的E -Z光异构化通过1 H NMR 进行定量,并通过超快激光光谱进行实时探测。光异构化过程发生在皮秒时间尺度上,导致荧光产率低。用增长弦法发现的多构型反应路径具有一个小障碍(4.3 或 6.5 kcal mol −1 ),处于 π – π *状态的E异构体必须克服该障碍才能达到连接E和2PyMIG 的Z异构体。虽然根据吡啶环的方向存在两种可能的路径,但两者沿路径和 MECI 表现出相同的定性特征,涉及 CCNN 和 CNNC 二面角的同时变化。 此外,吡啶环旋转的基态势垒很容易接近,因此两种 MECI 都存在通向实验观察到的Z异构体的低势垒途径,导致从E异构体到光产物的转变。将使用生长弦法的多构型反应路径计算与时间分辨荧光光谱相结合,为 2PyMIG 的光致异构化过程提供了重要的见解,导致计算和实验结果都表明通过单线态 π – π *和基态。
更新日期:2024-09-02
中文翻译:

从光谱方法和量子化学计算研究亚氨基胍受体的光异构化机制
腙官能团与吡啶基取代基结合时,提供了一类独特的广泛可调的光开关,其E - Z光异构化平衡可以通过N-H腙供体和吡啶基受体之间的分子内氢键来控制。然而,人们对这类化合物的光致异构化机制知之甚少。为了解决这个问题,我们报道了一种附加吡啶的亚氨基胍光开关,它在功能上与酰腙相关,并提供了对E和Z构型之间光异构化过程的深入了解。 E -2-吡啶基亚氨基胍阳离子 (2PyMIG) 在 DMSO(以溴化物盐形式制备)中的E -Z光异构化通过1 H NMR 进行定量,并通过超快激光光谱进行实时探测。光异构化过程发生在皮秒时间尺度上,导致荧光产率低。用增长弦法发现的多构型反应路径具有一个小障碍(4.3 或 6.5 kcal mol −1 ),处于 π – π *状态的E异构体必须克服该障碍才能达到连接E和2PyMIG 的Z异构体。虽然根据吡啶环的方向存在两种可能的路径,但两者沿路径和 MECI 表现出相同的定性特征,涉及 CCNN 和 CNNC 二面角的同时变化。 此外,吡啶环旋转的基态势垒很容易接近,因此两种 MECI 都存在通向实验观察到的Z异构体的低势垒途径,导致从E异构体到光产物的转变。将使用生长弦法的多构型反应路径计算与时间分辨荧光光谱相结合,为 2PyMIG 的光致异构化过程提供了重要的见解,导致计算和实验结果都表明通过单线态 π – π *和基态。

































 京公网安备 11010802027423号
京公网安备 11010802027423号