Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
From ex ovo to in vitro: xenotransplantation and vascularization of mouse embryonic kidneys in a microfluidic chip
Lab on a Chip ( IF 6.1 ) Pub Date : 2024-09-02 , DOI: 10.1039/d4lc00547c Micaela Oliveira 1 , Partha Protim Sarker 1, 2 , Ilya Skovorodkin 1, 2 , Ali Kalantarifard 1 , Tugce Haskavuk 1, 2 , Jonatan Mac Intyre 1 , Elizabath Nallukunnel Raju 1 , Samin Nooranian 1 , Hiroki Shioda 3 , Masaki Nishikawa 3 , Yasuyuki Sakai 3 , Seppo J Vainio 2, 4, 5 , Caglar Elbuken 1, 6 , Irina Raykhel 2, 3
Lab on a Chip ( IF 6.1 ) Pub Date : 2024-09-02 , DOI: 10.1039/d4lc00547c Micaela Oliveira 1 , Partha Protim Sarker 1, 2 , Ilya Skovorodkin 1, 2 , Ali Kalantarifard 1 , Tugce Haskavuk 1, 2 , Jonatan Mac Intyre 1 , Elizabath Nallukunnel Raju 1 , Samin Nooranian 1 , Hiroki Shioda 3 , Masaki Nishikawa 3 , Yasuyuki Sakai 3 , Seppo J Vainio 2, 4, 5 , Caglar Elbuken 1, 6 , Irina Raykhel 2, 3
Affiliation
|
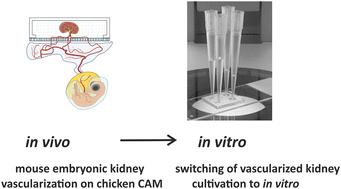
Organoids are emerging as a powerful tool to investigate complex biological structures in vitro. Vascularization of organoids is crucial to recapitulate the morphology and function of the represented human organ, especially in the case of the kidney, whose primary function of blood filtration is closely associated with blood circulation. Current in vitro microfluidic approaches have only provided initial vascularization of kidney organoids, whereas in vivo transplantation to animal models is problematic due to ethical problems, with the exception of xenotransplantation onto a chicken chorioallantoic membrane (CAM). Although CAM can serve as a good environment for vascularization, it can only be used for a fixed length of time, limited by development of the embryo. Here, we propose a novel lab on a chip design that allows organoids of different origin to be cultured and vascularized on a CAM, as well as to be transferred to in vitro conditions when required. Mouse embryonic kidneys cultured on the CAM showed enhanced vascularization by intrinsic endothelial cells, and made connections with the chicken vasculature, as evidenced by blood flowing through them. After the chips were transferred to in vitro conditions, the vasculature inside the organoids was successfully maintained. To our knowledge, this is the first demonstration of the combination of in vivo and in vitro approaches applied to microfluidic chip design.
中文翻译:

从卵外到体外:微流控芯片中小鼠胚胎肾的异种移植和血管化
类器官正在成为研究体外复杂生物结构的有力工具。类器官的血管化对于概括所代表的人体器官的形态和功能至关重要,尤其是在肾脏的情况下,其血液过滤的主要功能与血液循环密切相关。目前的体外微流控方法仅提供肾脏类器官的初始血管化,而由于伦理问题,体内移植到动物模型是有问题的,除了异种移植到鸡绒毛膜尿囊膜 (CAM) 上。虽然 CAM 可以作为良好的血管形成环境,但它只能在固定的时间长度内使用,受胚胎发育的限制。在这里,我们提出了一种新颖的芯片实验室设计,允许在 CAM 上培养和血管化不同来源的类器官,并在需要时转移到体外条件。在 CAM 上培养的小鼠胚胎肾显示内源性内皮细胞的血管形成增强,并与鸡脉管系统建立联系,血液流经它们证明了这一点。将芯片转移到体外条件后,类器官内的脉管系统得以成功维持。据我们所知,这是将体内和体外方法相结合应用于微流体芯片设计的首次演示。
更新日期:2024-09-02
中文翻译:

从卵外到体外:微流控芯片中小鼠胚胎肾的异种移植和血管化
类器官正在成为研究体外复杂生物结构的有力工具。类器官的血管化对于概括所代表的人体器官的形态和功能至关重要,尤其是在肾脏的情况下,其血液过滤的主要功能与血液循环密切相关。目前的体外微流控方法仅提供肾脏类器官的初始血管化,而由于伦理问题,体内移植到动物模型是有问题的,除了异种移植到鸡绒毛膜尿囊膜 (CAM) 上。虽然 CAM 可以作为良好的血管形成环境,但它只能在固定的时间长度内使用,受胚胎发育的限制。在这里,我们提出了一种新颖的芯片实验室设计,允许在 CAM 上培养和血管化不同来源的类器官,并在需要时转移到体外条件。在 CAM 上培养的小鼠胚胎肾显示内源性内皮细胞的血管形成增强,并与鸡脉管系统建立联系,血液流经它们证明了这一点。将芯片转移到体外条件后,类器官内的脉管系统得以成功维持。据我们所知,这是将体内和体外方法相结合应用于微流体芯片设计的首次演示。


















































 京公网安备 11010802027423号
京公网安备 11010802027423号